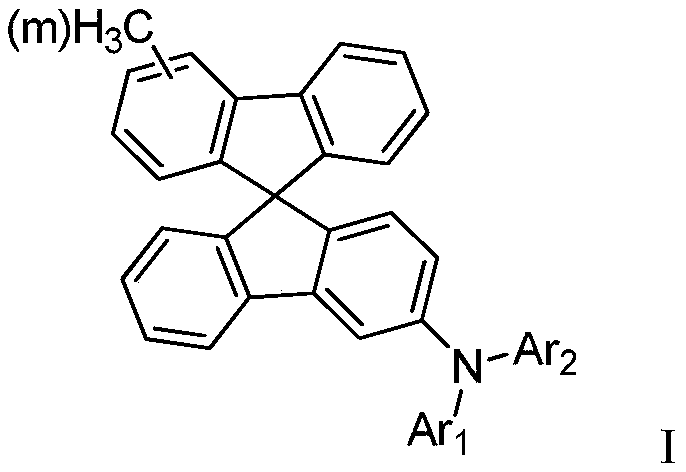
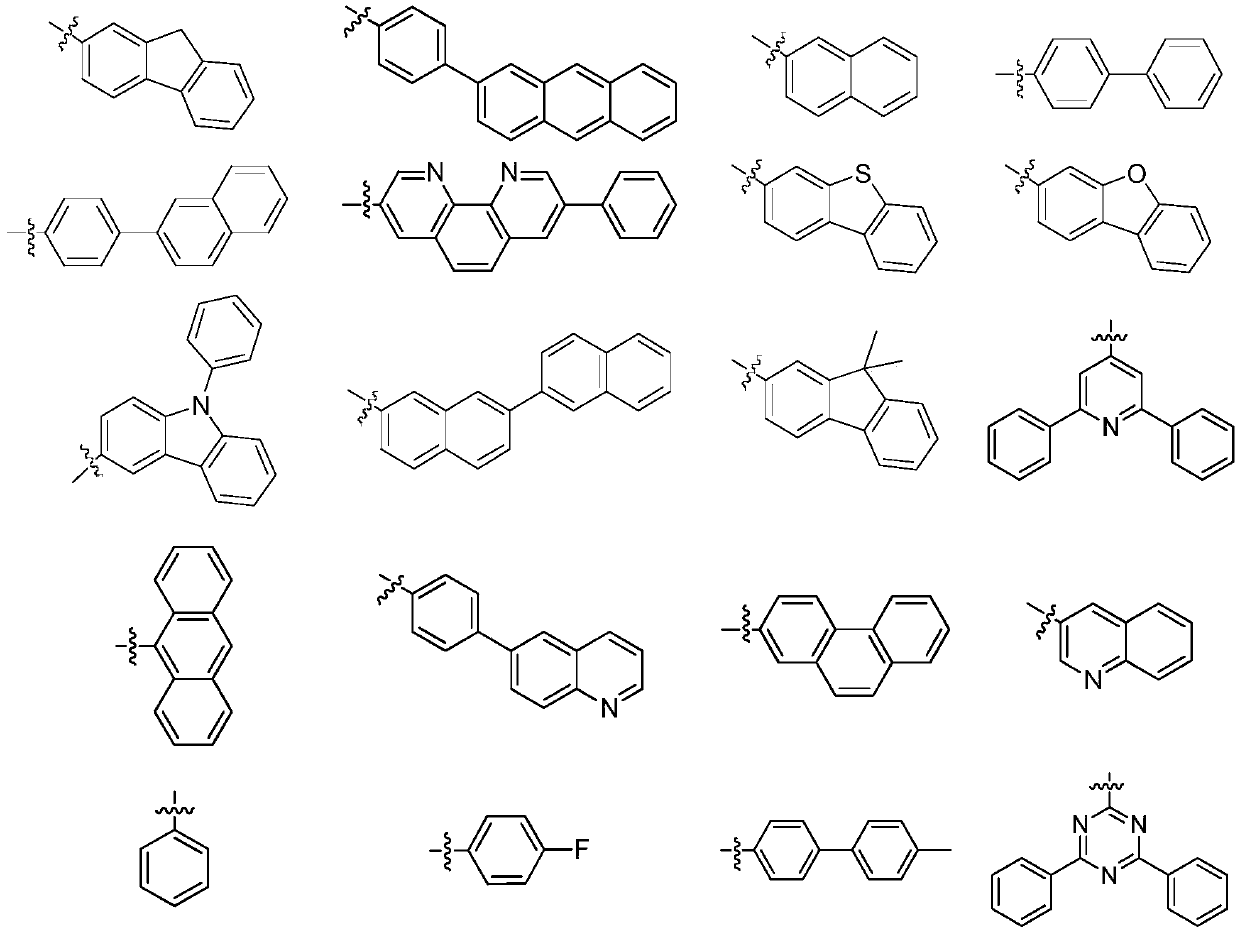
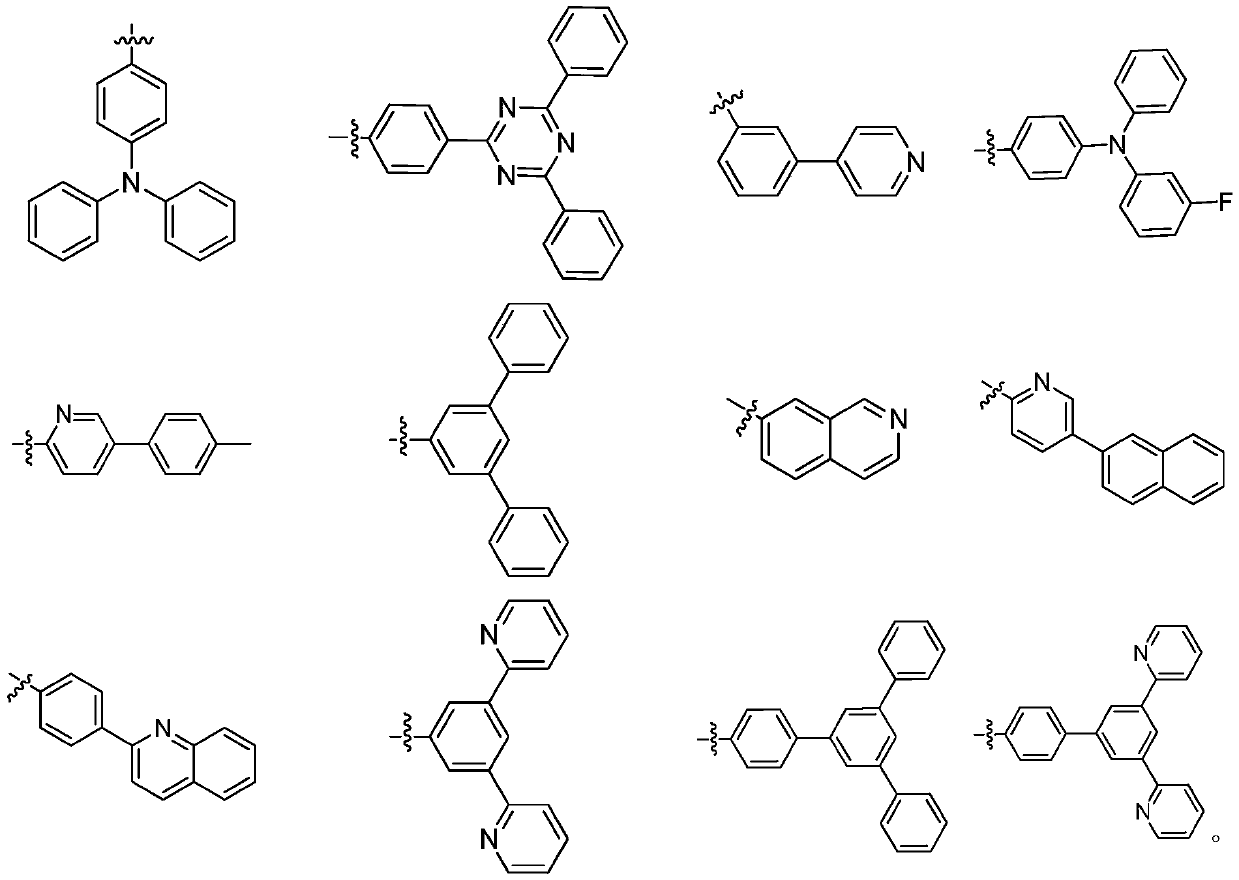
Organic compound as well as preparation method and application thereof
A technology of organic compounds and compounds, applied in organic chemistry, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., can solve problems such as low glass transition temperature, destruction of film uniformity, and impact on the service life of materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] (Compound I-2-1) Synthesis
[0069] The synthetic route is as follows:
[0070]
[0071] Including the following specific steps:
[0072] 500 ml three-neck flask, equipped with magnetic stirring, after argon replacement, add 18.1 g (0.188 mol) of potassium tert-butoxide, N-([1,1'-biphenyl]-4-yl)-9H-fluorene-2- Amine 33.34g (purity 99%, 0.1mol) and toluene 100ml. After nitrogen replacement again, 1.6 ml of tri-tert-butylphosphine and 0.23 g of palladium acetate were successively added. After the addition was complete, the temperature was raised to 85°C. Start to add dropwise a solution consisting of 48.82g of methylspirobifluorene-bromo-substituent-containing compound (A-2-1) (purity 99%, 0.1mol) and 100ml of toluene, and control the temperature at 80-120°C. Cool down to 50°C, add 100m deionized water to hydrolyze, stir for 10 minutes, filter, and boil the filter cake several times with DMF, and rotary steam to obtain 65.92g white solid with a purity of 99% and ...
Embodiment 2
[0075] Synthesis of (Compound I-31-1)
[0076] The synthetic route is as follows:
[0077]
[0078] Including the following specific steps:
[0079] 500 ml three-neck flask, equipped with magnetic stirring, after argon replacement, add 18.1 g (0.188 mol) of potassium tert-butoxide, N-dibenzo[b,d]thiophene-5-(p-tolyl)pyridin-2-amine in sequence 36.65g (purity 99%, 0.15mol) and toluene 100ml. After nitrogen replacement again, 1.6 ml of tri-tert-butylphosphine and 0.23 g of palladium acetate were successively added. After the addition was complete, the temperature was raised to 85°C. Start to add dropwise a solution consisting of 10.93g of methylspirobifluorene-bromo-substituent-containing compound (A-31-1) (purity 99%, 0.1mol) and 100ml of toluene, and control the temperature at 80-120°C. Cool down to 50°C, add 100m deionized water for hydrolysis, stir for 10 minutes, filter, and boil the filter cake several times with DMF to obtain 61.15g of white product with a purity...
Embodiment 3
[0082] Synthesis of (Compound I-55-1)
[0083] The synthetic route is as follows:
[0084]
[0085] Including the following specific steps:
[0086] 500 ml three-neck flask, equipped with magnetic stirring, after argon replacement, add 18.1 g (0.188 mol) of potassium tert-butoxide, N-(5-(p-tolyl)pyridin-2-yl)isoquinolin-7-amine in sequence 31.13g (99% purity, 0.15mol) and 100ml toluene. After nitrogen replacement again, 1.6 ml of tri-tert-butylphosphine and 0.23 g of palladium acetate were successively added. After the addition was complete, the temperature was raised to 85°C. Start to drop a solution consisting of 40.93g of the methylspirobifluorene-bromo-substituent-containing compound (A-55-1) (purity 99%, 0.1mol) and 100ml of toluene, and control the temperature at 80-120°C. Cool down to 50°C, add 100m deionized water for hydrolysis, stir for 10 minutes, filter, and boil the filter cake several times with DMF to obtain 57.90g of white solid with a purity of 99% an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com