A fluorescent probe for detecting butyrylcholinesterase activity and its preparation method and application
A butyrylcholinesterase and fluorescent probe technology, applied in the field of fluorescent probes, can solve problems such as no public detection function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
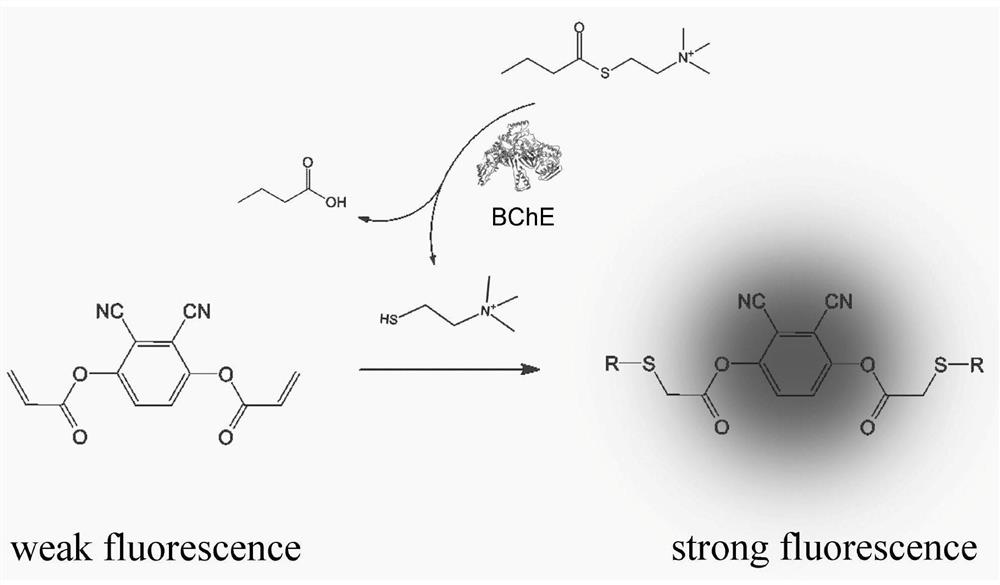
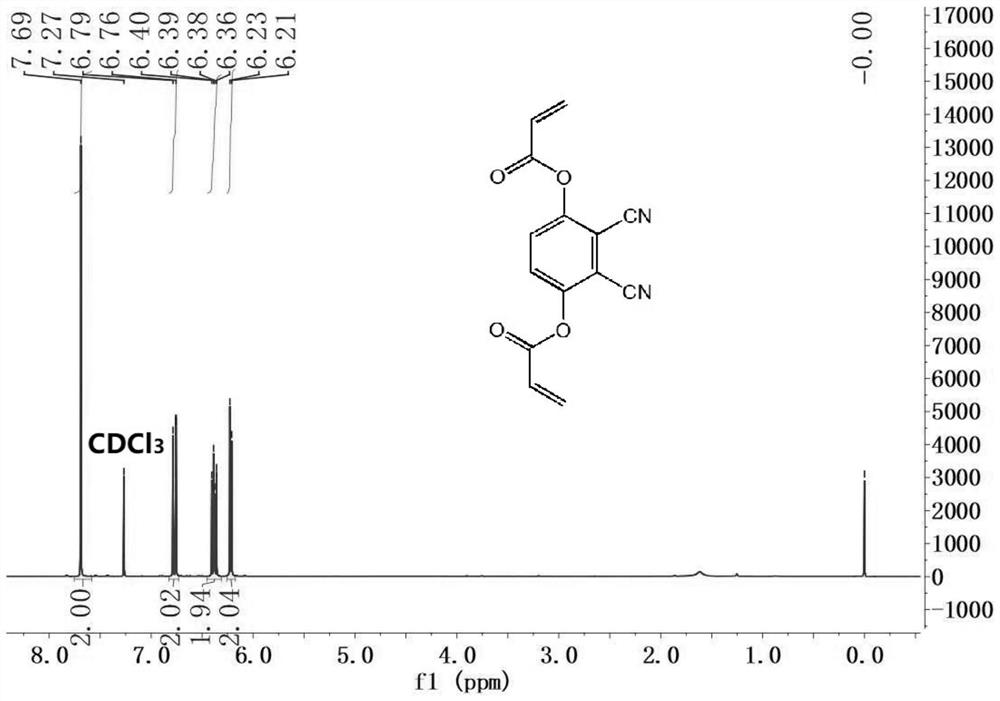
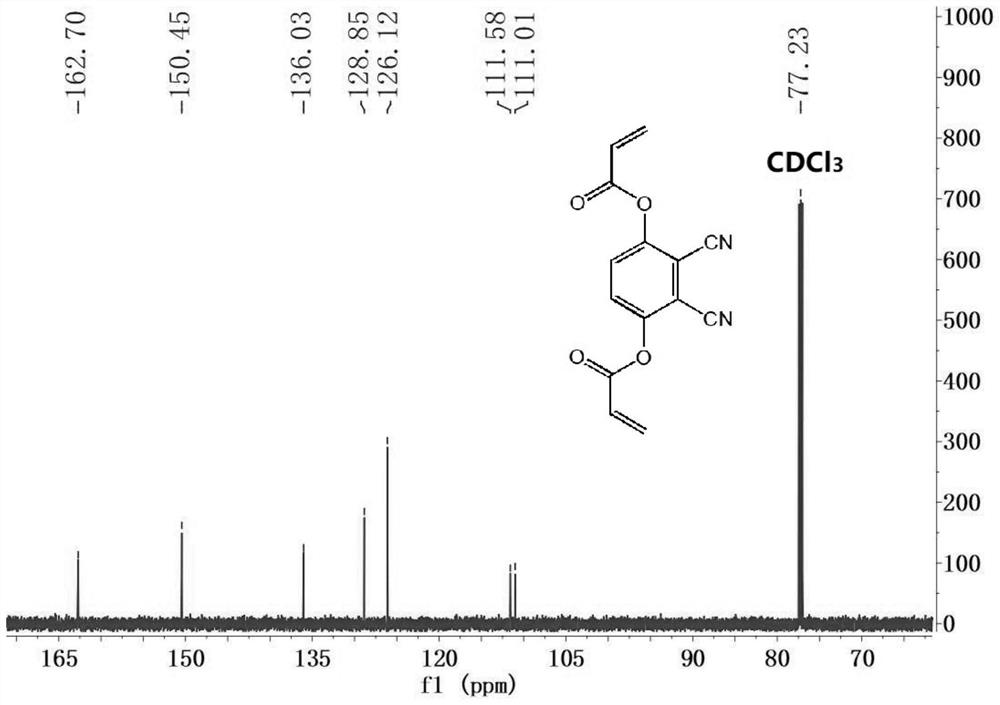
[0044] Two, 2,3-dicyano-1,4-phenylene diacrylate (DCPDA) to the fluorescence response of sulfhydryl compounds.
[0055] DCPDA, butyrylthiocholine and HEPES buffer. Apply the above fluorescence detection kit to butyrylcholinester
[0058] Except adding GSH at a concentration of 10.0 μM, 20.0 μM, 30.0 μM or 100.0 μM before adding 1.0 mM BTh,
[0061] In order to verify the function of BChE detection inhibitor screening, take tacrine as an example. Inhibition of BChE activity by tacrine
Embodiment 2
[0078] DCPDMA, butyrylthiocholine and HEPES buffer. Apply the above fluorescence detection kit to butyrylcholinester
[0079] First prepare a stock solution of DCPDMA solution in THF, wherein the DCPDMA solution is 10.0 mM; then mix 30.0 L
[0081] In addition to adding an amount of GSH (10.0 μM, 20.0 μM, 30.0 μM or 100.0 μM) prior to the addition of BTCh (1.0 mM)
[0082] The selective detection of BChE from mixtures with AChE was carried out as follows: containing specific levels of different species
[0083] In order to verify the function of BChE detection inhibitor screening, take tacrine as an example. Inhibition of BChE activity by tacrine
Embodiment 3
[0090] To 3.0 [mu]L of HEPES buffer was added 90 [mu]L of a 10.0 mM solution of DCPDFC. dissolved in the above DCPDFC
[0091] First prepare a stock solution of DCPDFC solution in THF, wherein the DCPDFC solution is 10.0 mM; then mix 30.0 μL
[0092] The experiment of the effect of GSH on the BChE assay was as follows: except that a certain
[0095] In order to verify the function of BChE detection inhibitor screening, take tacrine as an example. Inhibition of BChE activity by tacrine
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com