Quick construction method of CAR-T (chimeric antigen receptor T-cells) toxicity indictor cells
A technology for constructing methods and cells, applied in chemical instruments and methods, reverse transcription RNA viruses, botanical equipment and methods, etc., can solve the problems of large workload, low success rate, and long time-consuming indicator cells, so as to reduce costs, The effect of omitting preparation steps and saving preparation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] 1. Construction of lentiviral plasmid
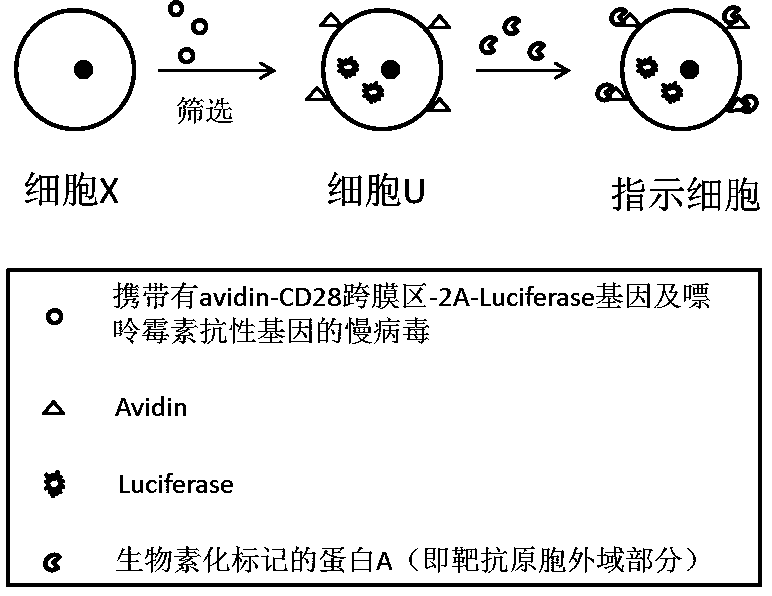
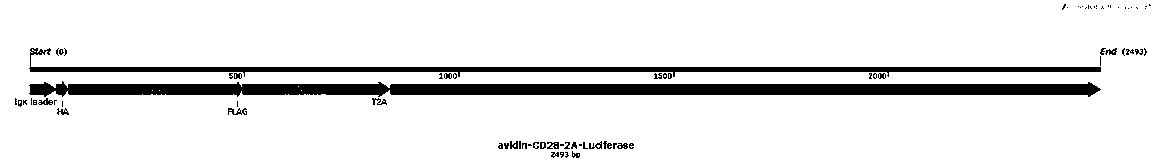
[0052] 1. Whole gene synthesis figure 1 The sequence of the gene fragment shown is SED NO: 1, which contains IgK leader, avidin, CD28 transmembrane region, T2A sequence and Luciferase derived from Firefly, and the upstream and downstream restriction sites are designed to be XhoI and BamHI respectively;
[0053] 2. Digest the above gene fragment and pLVX-IRES-Puro plasmid with XhoI and BamHI; cut the gel and recover, and connect with conventional molecular biology methods;
[0054] 3. Transform the ligation product into Stbl3 competent cells, and spread the LB medium plate (ampicillin resistance)
[0055] 4. Pick a single clone and culture it in LB medium for 8-12 hours;
[0056] 5. Small plasmid extraction, double enzyme digestion with XhoI and BamHI, combined with gel electrophoresis to verify whether the band size is correct, and further plasmid sequencing to verify the correctness with universal primers; the universal primers...
Embodiment 2
[0084] Construction of K562 cells carrying CD19 antigen
[0085] 1. Cultivate the K562-avidin-Luc cells constructed in step 3 in a 12-well plate so that they are in the logarithmic growth phase;
[0086] 2. See SEQ NO:2 for the amino acid sequence of the ectodomain of the CD19 antigen protein. This sequence can be entrusted to the company for exogenous expression. For easy purification, a histidine tag is added at the end of the sequence. After protein purification, entrust the company to carry out biotinylation treatment. Alternatively, the biotinylated CD19 ectodomain protein is commercially available and can be purchased, such as from ACROBiosystems.
[0087] 3. Take 1×10 6 To each K562-avidin-Luc cell, add the biotinylated CD19 antigen ectodomain protein obtained above to a final concentration of 10 μg / ml, and incubate at 4°C for 30 minutes;
[0088] 4. Centrifuge to remove the supernatant, resuspend with IMDM complete medium (containing 10% FBS), repeat this step once,...
Embodiment 3
[0090] Example 3 Construction of K562 cells carrying CD19 and CD30 antigens
[0091] 1. Cultivate the K562-avidin-Luc cells constructed in step 3 in a 12-well plate so that they are in the logarithmic growth phase;
[0092] 2. Prepare biotinylated CD19 and CD30 extracellular domain proteins;
[0093] 3. Take 1×10 6 K562-avidin-Luc cells, at the same time, add the biotinylated CD19 and CD30 ectodomain proteins to a final concentration of 10 μg / ml, and incubate at 4°C for 60 minutes;
[0094] 4. Centrifuge to remove the supernatant, resuspend with IMDM complete medium (containing 10% FBS), repeat this step once, and obtain K562 cells carrying CD19 and CD30 antigens.
[0095] The obtained K562 cells carrying CD19 and CD30 antigens stably express Luciferase and combine the extracellular domains of CD19 and CD30 antigens, which can be used as indicator cells for detecting the toxicity of CAR-T cells targeting both CD19 and CD30.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com