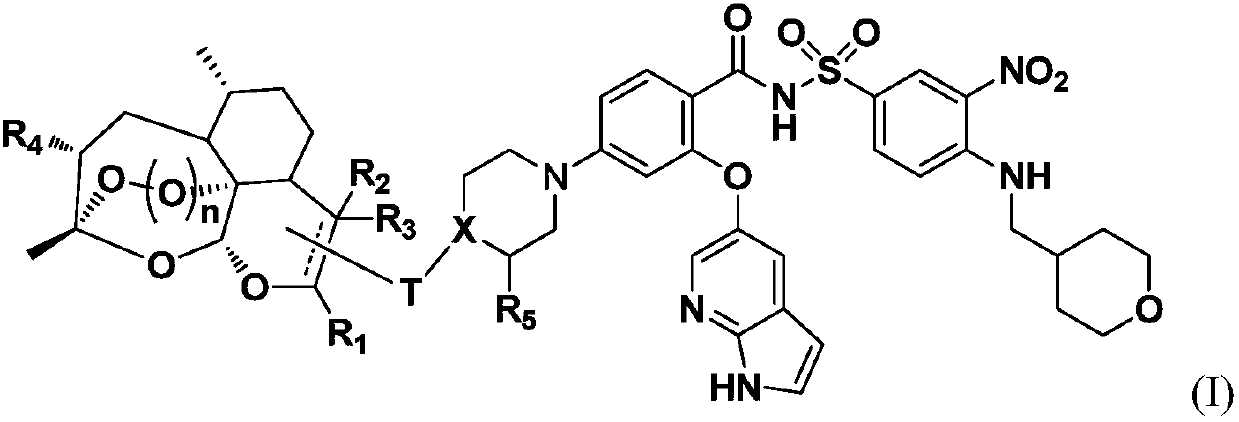
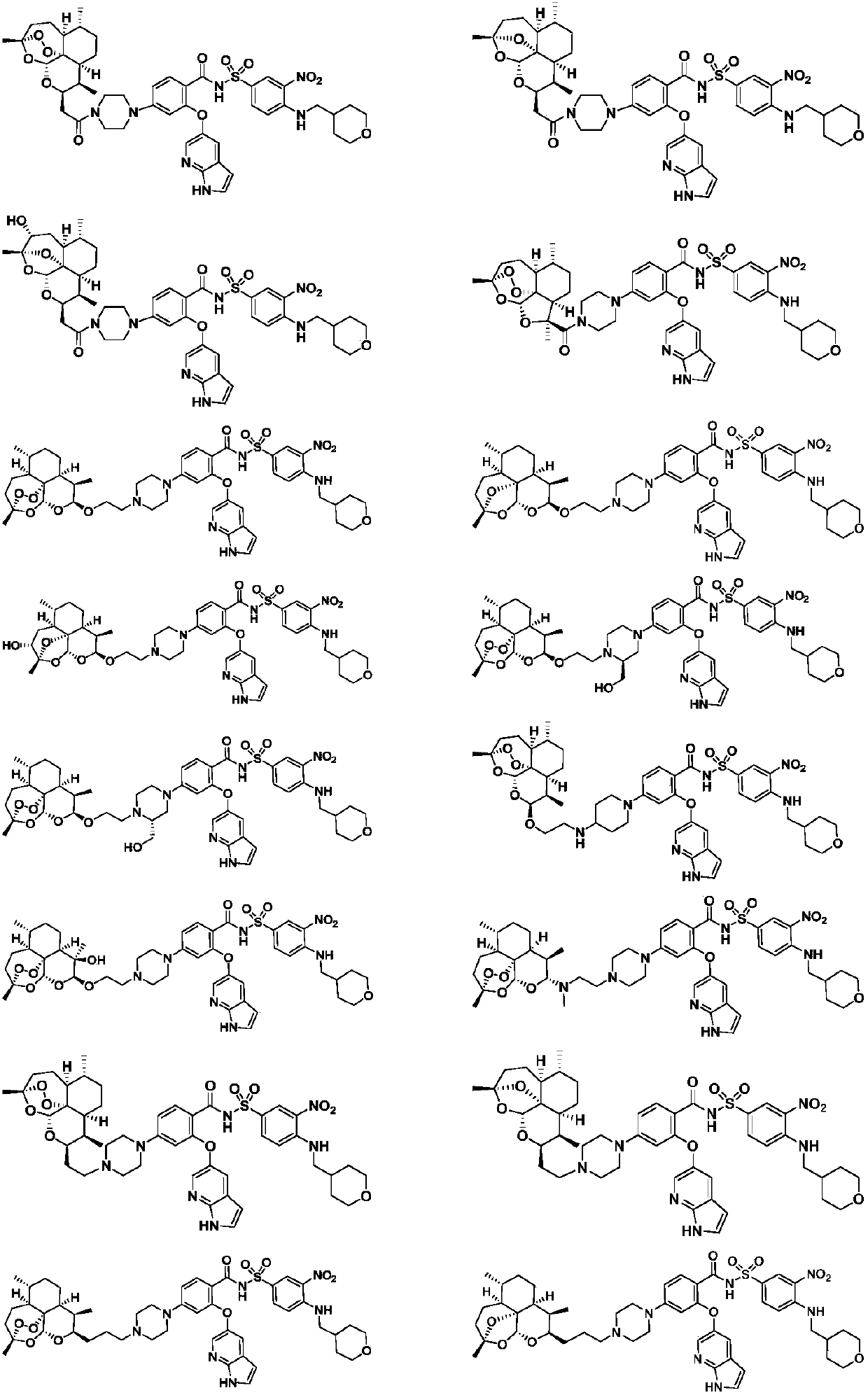
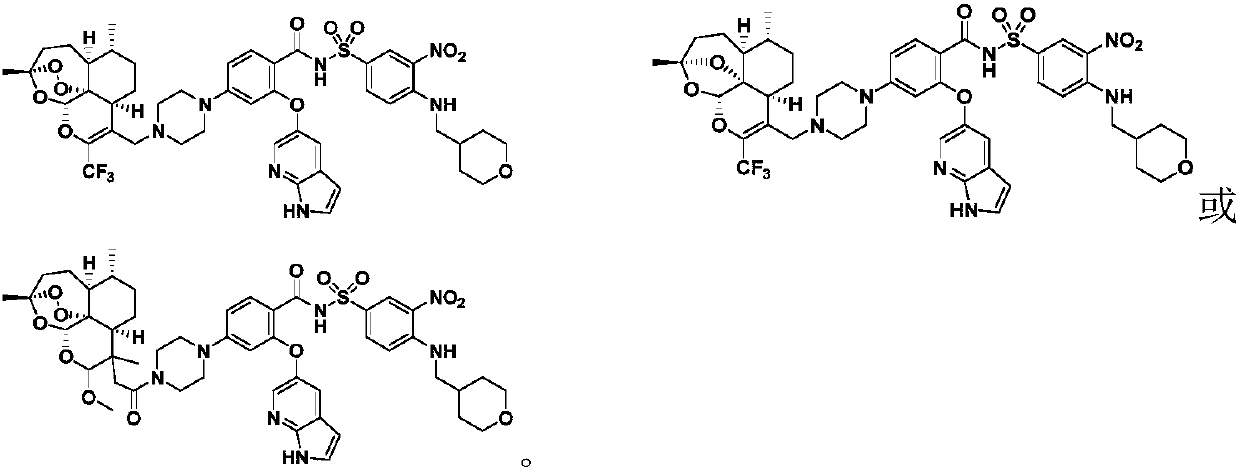
Novel artemisinin derivatives as well as synthetic method and application thereof
A condensation and halogen technology, applied in the field of new artemisinin derivatives, can solve problems such as poor water solubility and tumor lysis syndrome
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] The preparation of embodiment 1 compound S1
[0090]
[0091] The synthetic reference method of compound 1-1 (Org. Lett. 2005, 7, 1561-1564.).
[0092] The synthetic reference method of compound 1-2 (J. Med. Chem. 2008, 51, 6902-6915.).
[0093] Synthesis of Compound 1-3:
[0094] Dissolve compound 1-1 in dichloromethane, add (3eq) 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride [EDCI], (0.3eq) 4-di Methylaminopyridine [DMAP], after stirring at room temperature for half an hour, compound 1-2 (1.2eq) was added, followed by reaction at room temperature for 2-5 hours. After the reaction was complete, the reaction was quenched with water, extracted three times with dichloromethane, the combined organic phases were washed with saturated brine, dried over anhydrous sodium sulfate, and the sample was loaded on the column, CH 2 Cl 2 :MeOH=100:1~30:1 to obtain compound 1-3.
[0095] Synthesis of compounds 1-4:
[0096] Compound 1-3 was dissolved in EtOH:H 2...
Embodiment 2
[0101] The preparation of embodiment 2 compound S2
[0102]
[0103] Synthesis of compound 2-1:
[0104] Compound 1-3 was dissolved with glacial acetic acid [HOAc], and activated zinc powder (6eq) was added in batches, and stirred at room temperature for 3 days. After the reaction is complete, use sodium bicarbonate [NaHCO 3 ] solution was adjusted to pH = 7, extracted three times with ethyl acetate [EA], the combined organic phases were washed with saturated brine, dried over anhydrous sodium sulfate, and the sample was loaded on the column, CH 2 Cl 2 :MeOH=100:1~30:1 to obtain compound 2-1.
[0105] Synthesis of compound 2-2:
[0106] Compound 2-1 was dissolved in EtOH:H 2 To the mixed solvent of O=3:2, (2eq) lithium hydroxide was added, and stirred overnight at room temperature. After the reaction was complete, the reaction solution was spin-dried, dissolved by adding a small amount of water, then adjusted to pH = 6 with 1M aqueous HCl solution, and a white solid w...
Embodiment 3
[0110] The preparation of embodiment 3 compound S3
[0111]
[0112] Synthesis of Compound S3:
[0113] Compound S1 was dissolved in MeCN[acetonitrile]:H 2 In the mixed solvent of O=1:1, add (1.2eq) ferrous sulfate heptahydrate [FeSO 4 .7H 2 O], reacted at 37°C for 1 hour under the protection of nitrogen. After the reaction is complete, wash with water, extract three times with EA, combine the organic phases and wash with saturated brine, dry over anhydrous sodium sulfate, mix the sample and put it on the column, CH 2 Cl 2 :MeOH=100:1~40:1 to obtain compound S3.
[0114] 1 H NMR (400MHz, CDCl 3 )δ10.17(s,1H),9.80(s,1H),8.90(d,J=2.3Hz,1H),8.54(t,J=5.5Hz,1H),8.28–8.13(m,2H), 8.00(d, J=9.1Hz, 1H), 7.73(d, J=2.3Hz, 1H), 7.49(t, J=2.8Hz, 1H), 6.92(d, J=9.2Hz, 1H), 6.62– 6.50(m,2H),6.00(d,J=2.3Hz,1H),5.10(s,1H),4.59(q,J=7.2Hz,1H),4.03(dd,J=11.4,3.7Hz,2H ),3.83(d,J=13.5Hz,1H),3.62(s,1H),3.54–3.36(m,5H),3.31–3.03(m,6H),2.57(dd,J=14.9,8.5Hz, 1H), 2.43–2.29(m, 2H), 2.18(s,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com