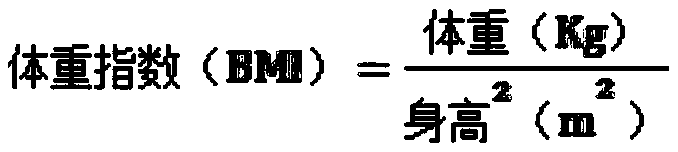Dietary substance supplementing composition, chewable tablets and preparation method and application of chewable tablets
A technology of composition and chewable tablet, which is applied in the field of dietary supplement composition, can solve problems such as physical hazards, and achieve the effects of lowering blood sugar and weight management
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The present invention also provides a preparation method for the above-mentioned chewable tablet, which comprises pulverizing and mixing the dietary supplement composition and auxiliary materials, and compressing the obtained mixture into tablets to obtain a chewable tablet.
[0026] In the present invention, after the dietary supplement composition and auxiliary materials are pulverized, the obtained pulverized material is sieved and then mixed, and the mesh size of the sieve used for the sieving is preferably 30-60 mesh, more preferably 40 mesh. ~50 mesh, most preferably 45 mesh. The present invention has no special limitation on the mixing method, and it is preferred to use equal-incremental mixing. The equal-incremental mixing refers to taking a small-content component and an equal amount of a large-content component, mixing them uniformly, and then adding an equal amount of the same mixture The large content components are mixed evenly, and the amount is increased ...
Embodiment 1
[0034] 40g of white kidney bean extract containing 55% α-amylase inhibitor, 30g of sorbitol, 21.5g of resistant dextrin, 2g of L-arabinose, 0.5g of magnesium stearate, and oat flour were passed through a 30-mesh sieve. 3g, 2g of xylooligosaccharide and 1g of maltitol are mixed by a mixer in an equal increasing manner, and then compressed with a tablet machine, with a total of 140 tablets, and the obtained chewable tablets are packaged with a packaging machine. Nitrogen is maintained during the process to keep the active ingredients stable.
Embodiment 2
[0036] 35g of white kidney bean extract containing 55% α-amylase inhibitor, 35g of sorbitol, 20g of resistant dextrin, 3g of L-arabinose, 0.5g of α-cyclodextrin, hard Magnesium fatty acid 0.5g, fructooligosaccharides 5.98g and steviol glycosides 0.02g are mixed by a blender in an equal increasing manner, and then compressed with a tablet machine, and 140 tablets are compressed altogether, and the chewable tablets obtained are packed with a packaging machine Carry out packing, keep nitrogen filling during the whole packing process, keep active ingredient stable.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com