Cepharanthine nanosuspension and preparation method thereof
A nano-suspension, the technology of Stephalin, which is applied in the field of medicine, can solve the problems of not improving the dissolution rate and bioavailability of stepherin, not eliminating vascular irritation, and poor patient compliance, so as to improve the oral Bioavailability, increased specific surface area, and enhanced steric hindrance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the preparation of stepahelin nanosuspension
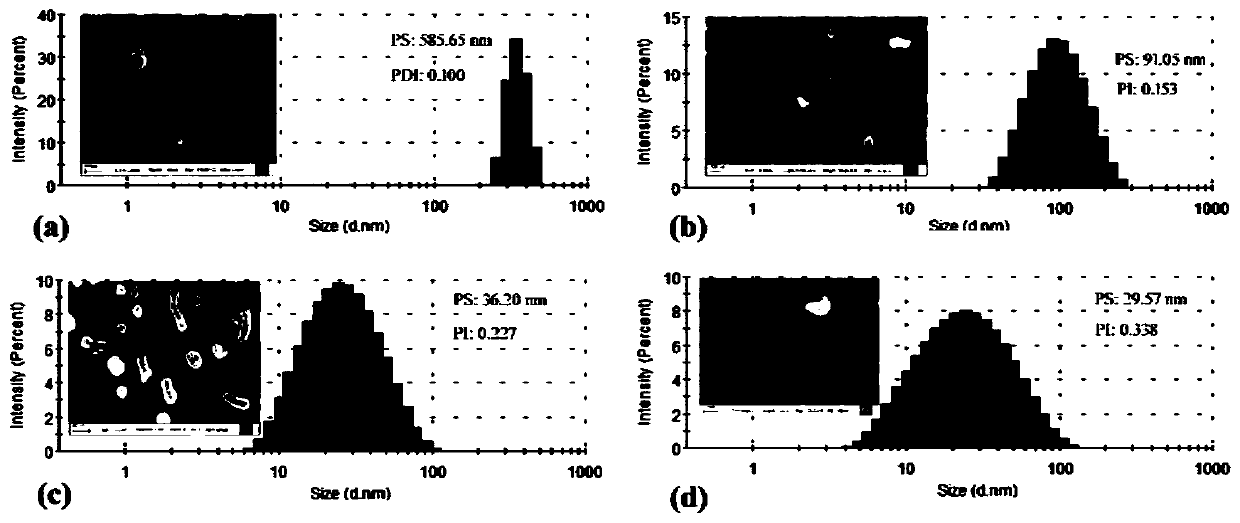
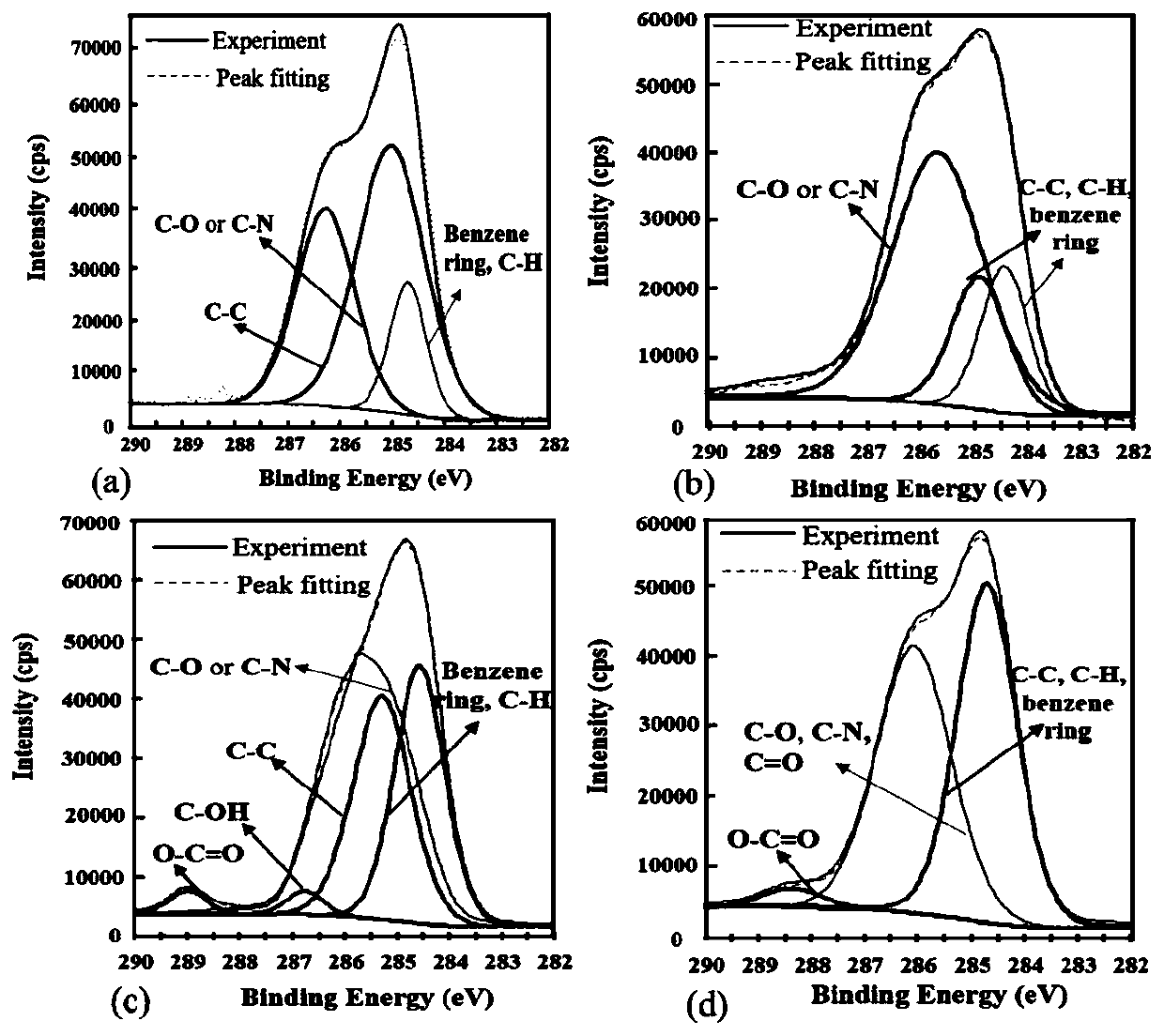
[0043] Weigh 0.1 g of the stephenin raw material drug, add it into 50 mL of distilled water, stir evenly to obtain an aqueous stabilizer solution, and stir under magnetic stirring for 20 minutes to obtain a coarse suspension. Add 140 mL of grinding media (specification d=0.6-0.8 mm) into the grinding chamber of the sand mill, transfer the coarse suspension to the grinding chamber, and grind for 45 min at 2000 rpm to obtain rod-shaped nanomixed particles with a particle size of 585.65 nm. Suspension ( figure 1 a), XPS is the surface element analysis of patinol ( image 3 a), the crystalline state of the drug as Figure 5 a and Figure 6 a shows obvious characteristic peaks.
Embodiment 2
[0044] Embodiment 2: the preparation of paternogenin nanosuspension
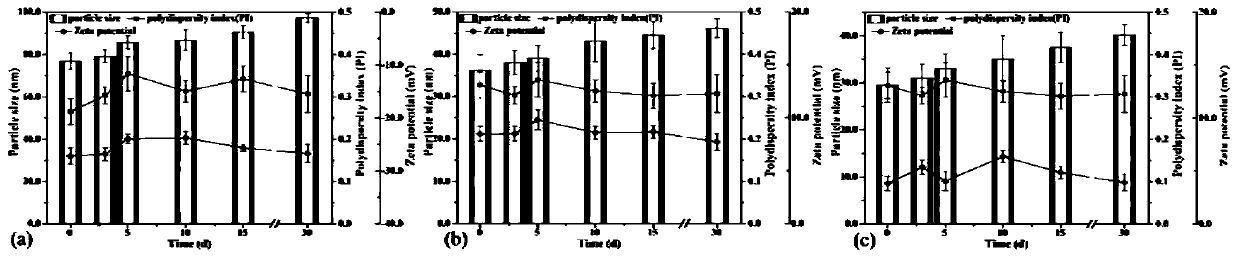
[0045]Weigh 0.1 g of vitamin E polyethylene glycol 1000 succinate, add 50 mL of distilled water, and stir evenly to obtain an aqueous stabilizer solution, add 0.5 g of stepherin raw material under magnetic stirring, and stir for 20 minutes to obtain a coarse suspension. Add 140 mL of grinding media (specification d=0.6-0.8 mm) into the grinding chamber of the sand mill, transfer the coarse suspension to the grinding chamber, and grind for 45 min at 2000 rpm to obtain a particle size of 36.2 nm ( figure 1 c), a rod-shaped nanosuspension at a potential of 6.34 mV ( figure 2 b), XPS analysis shows that the stable nanosuspension of TPGS shows the decrease of the N element of the raw material drug and the adsorption amount of TPGS on the surface of the nanosuspension particles determined by TGA, all confirming that the carrier covers the surface of the nanoparticles ( image 3 c), ( Figure 4 ). In addition, ...
Embodiment 3
[0046] Embodiment 3: the preparation of stepahelin nanosuspension
[0047] Weigh 0.03g of sodium alginate and 0.01g of sodium lauryl sulfate, add 50mL of distilled water, and stir evenly to obtain an aqueous stabilizer solution, add 0.8g of stephadin raw material under magnetic stirring, and stir for 20 minutes to obtain a coarse suspension liquid. Add 140 mL of grinding medium (specification d=0.6-0.8 mm) into the grinding chamber of the sand mill, transfer the coarse suspension to the grinding chamber, and grind for 45 min at 2030 rpm to obtain an opalescent nanosuspension.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com