Nasal composition and application thereof
A composition and a technique for nasal use, which are applied in the directions of drug combinations, plant raw materials, and medical preparations containing active ingredients, etc., can solve the problems that drugs for rhinitis are difficult to reach above the nasal cavity, difficult to treat, and inaccurate in curative effect, etc. The method is simple and easy to implement, and the effect of improving nasal discomfort and improving medication compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4
[0095] The nasal cavity drops were prepared according to the composition and content shown in Table 2 by conventional methods, and the content in Table 2 represented wt%.
[0096] Ingredient composition and content of the nasal cavity drops in table 2, embodiment 1~4
[0097]
[0098]
experiment example 2
[0105] Evaluation of nasal drip:
[0106] The evaluation index of nasal drip is divided into four items: "bitterness", "sweetness", "stimulation" and "stimulation duration", and the evaluation indicators of each item are from weak to strong (stimulation duration is stimulation time From short to long) are divided into 0, 1, 2, 3, 4 and 5 points, subdivided into "no feeling to weak" is 0 to 1 point, "moderate to strong" is 2 to 3 points, "strong to strong" Violent" is 4 to 5 points.
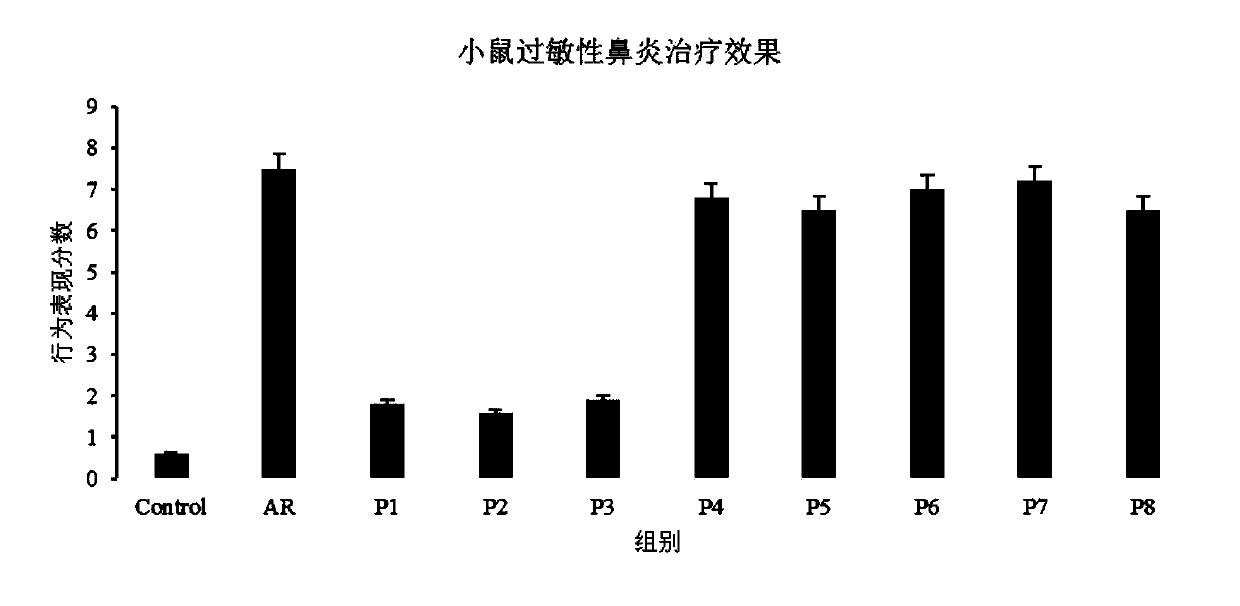
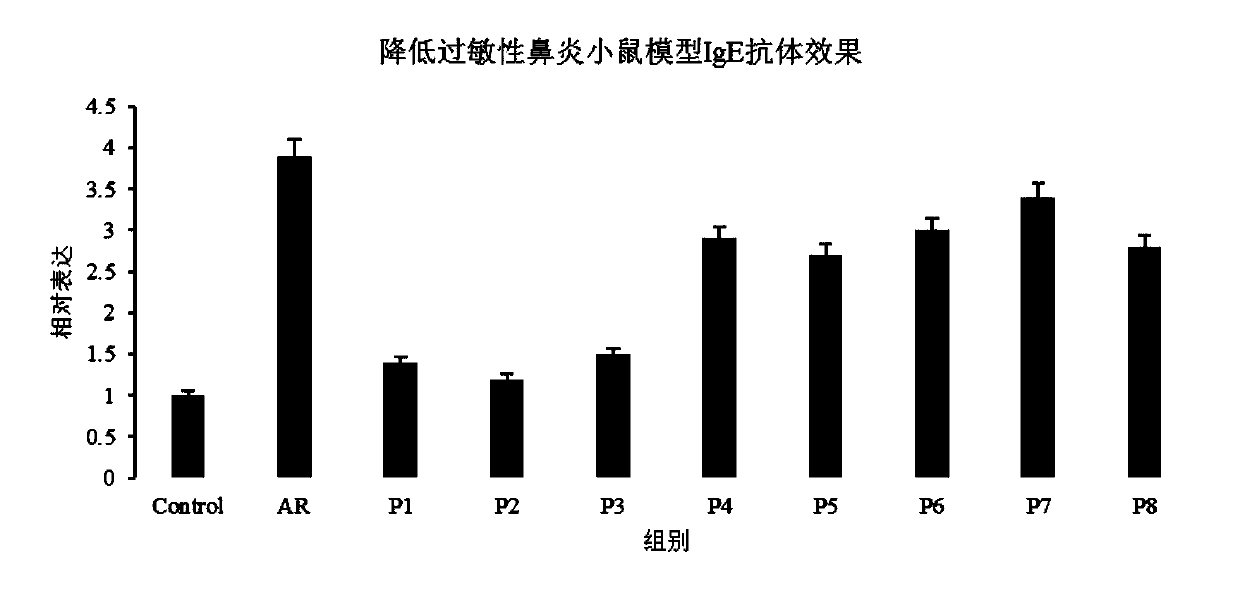
[0107] Each nasal cavity drop in Examples 1 to 4 and Comparative Examples V5 to V8 is filled in the drop container, and after spraying into the noses of 20 volunteers for 10 seconds, the nasal drop sensation evaluation test for the above items is carried out. The test results are shown in Table 4 below, and the results in Table 4 represent the average value of each evaluation score.
[0108] Table 4. Statistics of nasal dripping evaluation
[0109] Example Bitterness Sweetness e...
Embodiment 9
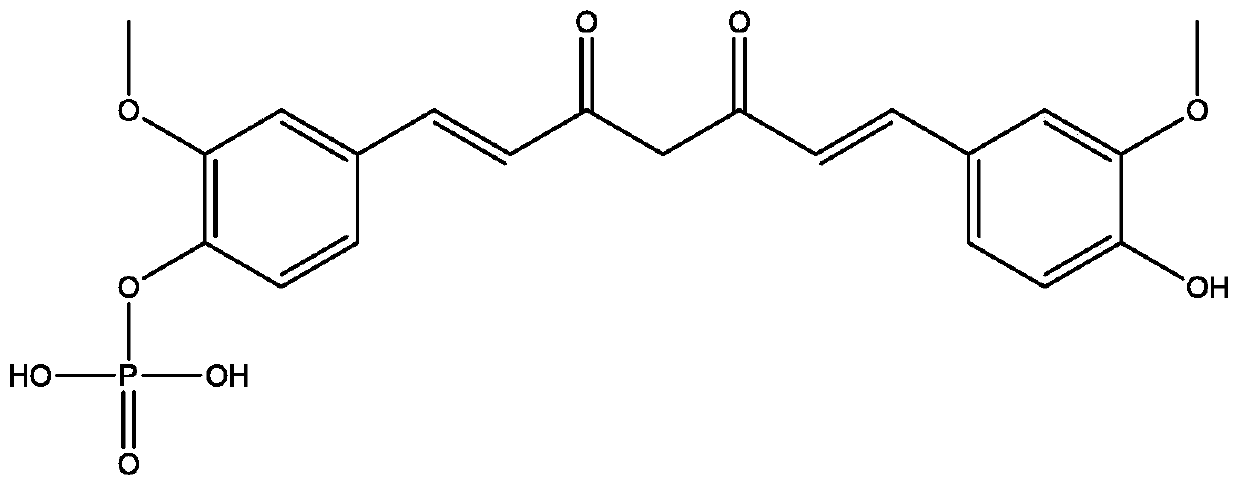
[0112] This embodiment provides a kind of curcumin monophosphate monosodium salt, dissolve 1.0g curcumin monophosphate in 300mL methanol, add 0.13g sodium methoxide, stir and react at room temperature for 1h, then evaporate the solvent under reduced pressure, and the residue is Dissolve in water: acetonitrile (1:8), place to crystallize, and filter to obtain the crystalline curcumin monophosphate monosodium salt.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com