Application of protein C in treating hepatic portal hypertension
A technique of arterial hypertension and hepatic portal, applied in the application field of protein C in the treatment of hepatic portal hypertension-related diseases, can solve the problems of lack of research on the pathogenesis and treatment mechanism of portal hypertension, and achieve tissue reduction and lower portal hypertension. Pulse pressure, the effect of promoting apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
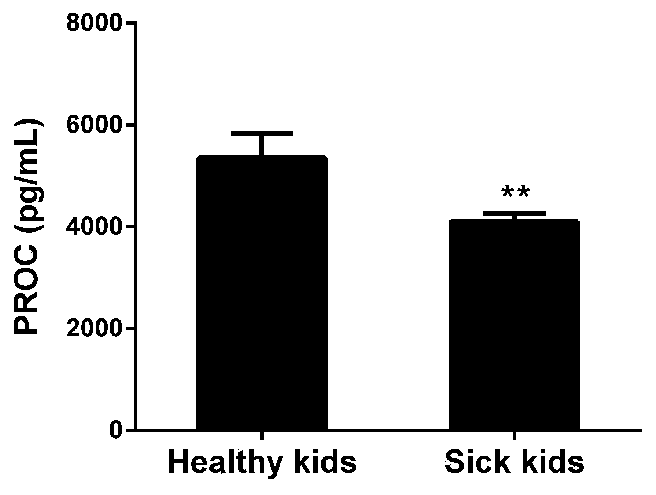
[0031] Example 1. Detection of relative content of activated protein C (PROTEIN C, PROC).
[0032] 1. Experimental group
[0033] Control group: whole blood samples from 6 normal children
[0034] Experimental group: Whole blood samples from 6 children with low protein C levels after portal vein cavernous degeneration Rex operation
[0035] 2. ELISA experiment
[0036] Set blank wells, standard wells and sample wells to be tested respectively. Blank wells (do not add samples, enzyme-labeled reagents and biotin-labeled anti-PROTEIN C antibodies, and the rest of the steps are the same), add 50 μL of standard products to the standard wells, add 400 μL of the sample to be tested, and then add biotin-labeled Anti-MCP3 antibody 10μL, shake gently to mix. The experimental steps were carried out according to the ELISA experimental operating procedures. The optical density (OD value) of each well was measured with a microplate reader at a wavelength of 450 nm.
[0037] 3. Experim...
Embodiment 2
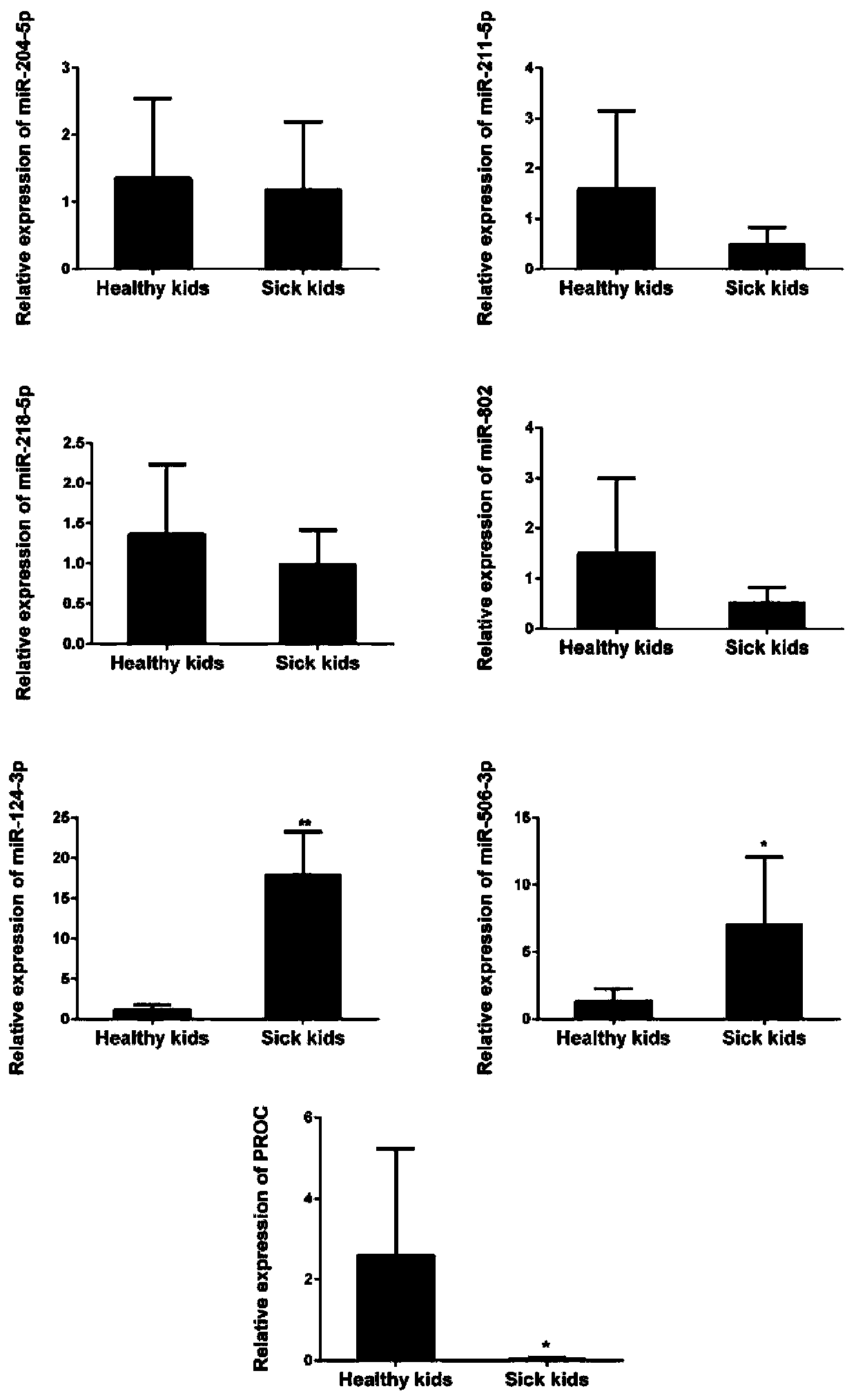
[0039] Embodiment 2, detect the relative content of miRNA and PROC mRNA
[0040] 1. Primer design
[0041] Table 1 Primer Sequence
[0042]
[0043]
[0044] 2. Extraction of RNA
[0045]1. Use TRI REAGENT BD kit to extract RNA in plasma, add 0.25mL plasma and 1-4μL polyacrylamide carrier to TRI reagent, mix thoroughly and place at room temperature for 5min.
[0046] 2. Add 0.2mL chloroform or 0.1mL BCP to each TRI reagent, shake well for 15s, let stand at room temperature for 2-5min, centrifuge at 12000g for 15min at 4°C, and absorb the upper aqueous phase.
[0047] 3. Add 0.5mL isopropanol to each TRI reagent and mix well, place at room temperature for 5-10min, centrifuge at 12000g for 10min at 4°C, discard the supernatant, and the RNA will sink to the bottom of the tube.
[0048] 4. Add 1mL of 75% ethanol to each TRI reagent, shake the centrifuge tube, suspend the precipitate, centrifuge at 7500g at 4°C for 5min, discard the supernatant, and dry at room temperature...
Embodiment 3
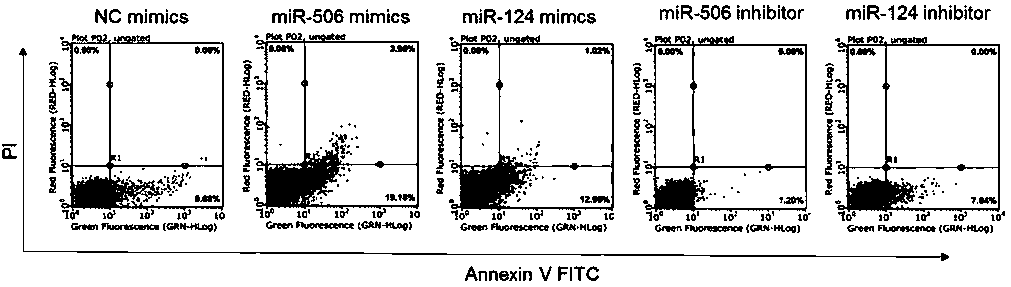
[0076] Embodiment 3, verify the double luciferase experiment of PROC target gene
[0077] 1. Experimental group
[0078] (1) NC mimics+Luc-PROCwt. (2) miR-506mimic+Luc-PROCwt. (3) miR-124mimic+Luc-PROCwt. (4) NC mimics+Luc-PROCmut. (5) miR-506mimic+Luc-PROCmut. (6) miR-124mimic+Luc-PROCmut.
[0079] After miRNA mimics, firefly luciferase and Renilla luciferase plasmids were co-transfected into 293T cells for 48 hours, Luciferase activity was detected.
[0080] 2. Experimental steps
[0081] 1. Discard the medium, wash with 1x PBS, and discard the PBS.
[0082] 2. Add 100 μL 1x PLB (1 volume of 5X Passive Lysis Buffer plus 4 volumes of double-distilled water) to each well, and place on a shaker at room temperature for 15 minutes.
[0083] 3. After the cells are fully lysed, add the lysate to the 96-well plate, and add 20 μL to each sample.
[0084] 4. Add 100 μL LAR II to each well of the sample to be tested (use Luciferase Assay Buffer II to resuspend Luciferase Assay...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com