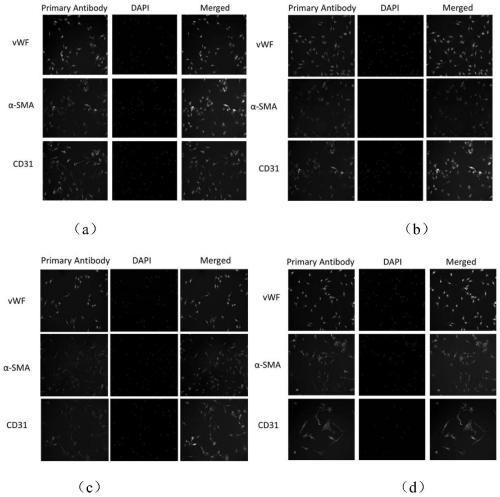
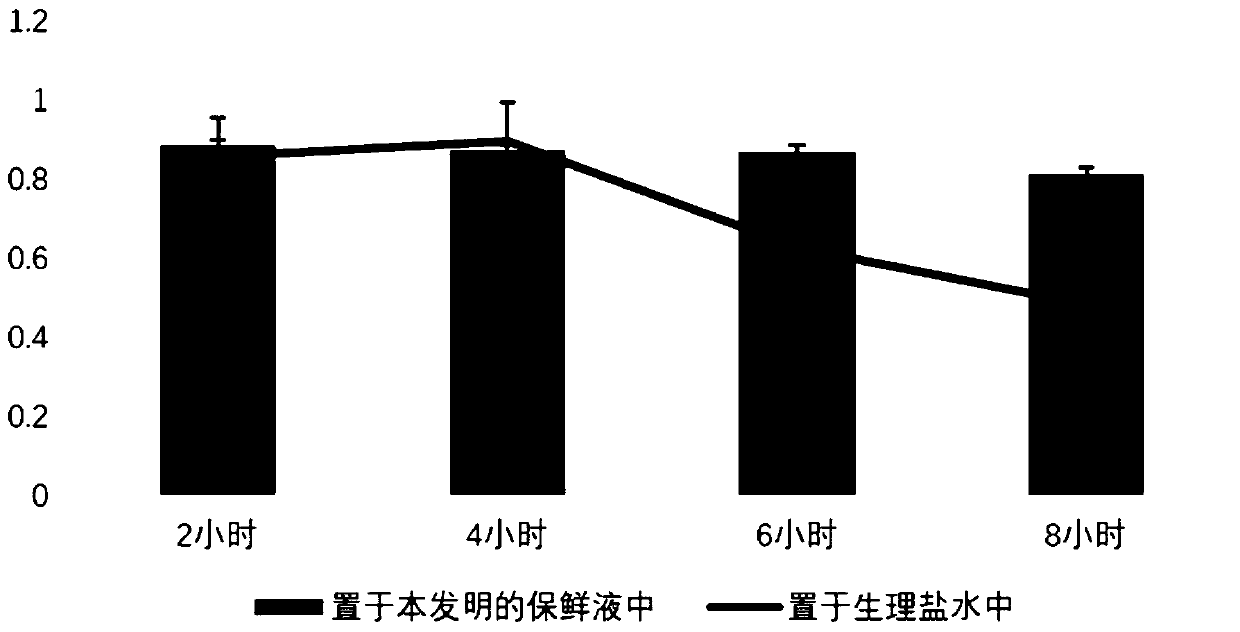
Primary isolation and culture method of human umbilical vein endothelium
A technology for separating and culturing umbilical veins, applied in the biological field, can solve the problems of various reagents, decreased cell viability, aging, shrinking and death, etc., to avoid vascular smooth muscle shedding, improve feasibility, and good cell viability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] ①Umbilical cords of healthy full-term fetuses were collected. Before the umbilical cord is collected, it is necessary to review the results of the maternal examination to exclude the maternal with infectious diseases (AIDS, hepatitis B, hepatitis C, syphilis, etc.) to ensure the safety of the experimental operators;
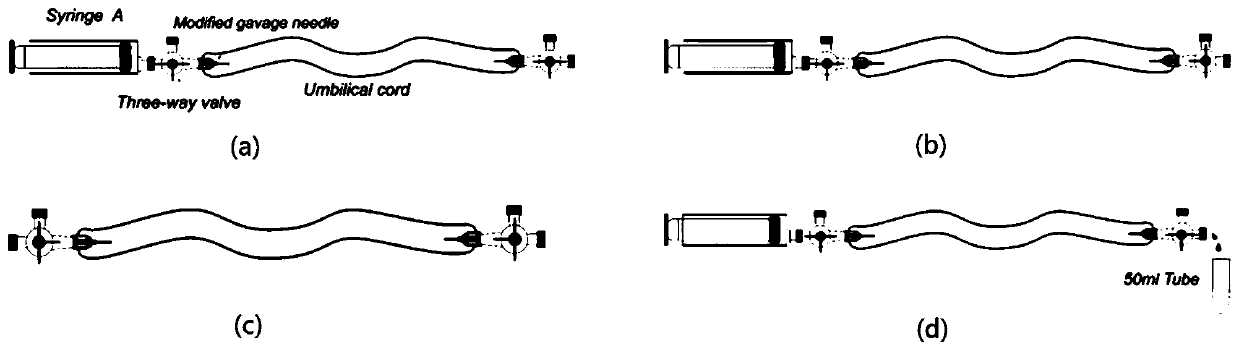
[0063] ②Take a 15cm-long umbilical cord in a sterile environment, cut off the hemostatic clamp marks at both ends of the umbilical cord, gently squeeze out the residual blood in the blood vessel, put the umbilical cord into the storage solution, place it on ice and transport it back to the laboratory;
[0064] ③Add 3mL 0.1% gelatin solution to the T25 culture flask, put it in 36.5℃, CO 2 4.5% concentration in the incubator for 30 minutes, pour off the gelatin solution before inoculation, and rinse the wall of the culture bottle with 2mL sterile PBS solution;
[0065] ④Transfer the umbilical cord to the sterilized waist plate in the ultra-clean bench, wipe...
Embodiment 2
[0071] ①Collect the umbilical cord of a healthy full-term fetus; before the collection of the umbilical cord, it is necessary to review the results of the maternal examination and exclude the maternal with infectious diseases (AIDS, hepatitis B, hepatitis C, syphilis, etc.) to ensure the safety of the experimental operators;
[0072] ②Take a 20cm-long umbilical cord in a sterile environment, cut off the hemostatic clamp marks at both ends of the umbilical cord, gently squeeze out the residual blood in the blood vessel, put the umbilical cord into the storage solution, place it on ice and transport it back to the laboratory;
[0073] ③Add 3mL 0.1% gelatin solution to the T25 culture flask, put it in 37.5℃, CO 2The concentration is 5.5% in the incubator for 30 minutes, pour off the gelatin solution before inoculation, and rinse the wall of the culture bottle with 2mL PBS solution;
[0074] ④Transfer the umbilical cord to the sterilized waist plate in the ultra-clean bench, wipe ...
Embodiment 3
[0080] ①Collect the umbilical cord of a healthy full-term fetus; before the collection of the umbilical cord, it is necessary to review the results of the maternal examination and exclude the maternal with infectious diseases (AIDS, hepatitis B, hepatitis C, syphilis, etc.) to ensure the safety of the experimental operators;
[0081] ②Take a umbilical cord with a length of 30 cm under a sterile environment, cut off the hemostatic clamp marks at both ends of the umbilical cord, gently squeeze out the residual blood in the blood vessel, put the umbilical cord into the storage solution, place it on ice and transport it back to the laboratory;
[0082] ③ Add 3mL 0.1% gelatin solution to the T25 culture flask, put it in 37℃, CO 2 The concentration is 5% in the incubator for 30 minutes, pour off the gelatin solution before inoculation, and rinse the wall of the culture bottle with 2mL PBS solution;
[0083] ④Transfer the umbilical cord to the sterilized waist plate in the ultra-clea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com