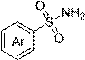
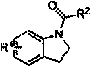Method for preparing N-arylsulfonamide from indoline and arylsulfonamide
A technology of arylsulfonamide and indoline, which is applied in the field of C-H bond activation, can solve the problems of restricting large-scale use and generating a large amount of waste, and achieves the effects of short reaction time, easy-to-obtain catalyst and wide applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
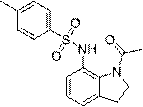
[0022] Example 1: Preparation of N-(1-acetylindolin-7-yl)-4-methylbenzenesulfonamide N-(1-acetylindolin-7-yl)-4-methylbenzenesulfonamide
[0023]
[0024] In the dried Schlenk tube, the raw materials indoline (0.15 mmol, 24.1 mg), p-toluenesulfonamide (0.3 mmol, 51.1 mg), iodobenzene acetate (0.15 mmol, 48.3 mg), pentamethyl Cyclopentadienyl rhodium dichloride (5 mmol%, 4.6 mg), additives, silver trifluoromethanesulfonate (20 mmol%, 7.7 mg), silver acetate (20% mmol, 5.1 mg), acetic acid (0.45 mmol, 27 mg) 1.5 mL of 1,2-dichloroethane, and put the above-mentioned Schlenk tube at 100 o In a C oil bath, stir for about 16 h. The reaction was terminated, and the reaction solution was easily quenched with 2 mL of saturated ammonium chloride, and extracted several times with ethyl acetate (4 mL × 5), the organic phases were combined, and the solvent was removed on a rotary evaporator. Finally, separated by silica gel column chromatography (eluent: ethyl acetate: petroleum ether...
Embodiment 2
[0026] Example 2: Preparation of N-(1-acetyl-2-methylindolin-7-yl)-4-methylbenzenesulfonamide N-(1-acetyl-2-methylindolin-7-yl)-4 -methylbenzenesulfonamide
[0027]
[0028] In the dried Schlenk tube, add the raw materials indoline (0.15 mmol, 26.2 mg), p-toluenesulfonamide (0.3 mmol, 51.1 mg), iodobenzene acetate (0.15 mmol, 48.3 mg), pentamethyl Cyclopentadienyl rhodium dichloride (5% mmol, 4.6 mg), additives, silver trifluoromethanesulfonate (20% mmol, 7.7 mg), silver acetate (20% mmol, 5.1 mg), acetic acid (0.45 mmol, 27 mg) 1.5 mL of 1,2-dichloroethane, and put the above-mentioned Schlenk tube at 100 o In a C oil bath, stir for about 16 h. The reaction was terminated, and the reaction solution was easily quenched with 2 mL of saturated ammonium chloride, and extracted several times with ethyl acetate (4 mL × 5), the organic phases were combined, and the solvent was removed on a rotary evaporator. Finally, it was separated by silica gel column chromatography (eluent:...
Embodiment 3
[0030] Example 3: Preparation of N-(1-acetyl-2,3,3-ylindoline-7-yl)-4-methylbenzenesulfonamide N-(1-acetyl-2,3,3-trimethylindolin -7-yl)-4-methylbenzenesulfonamide
[0031]
[0032] In the dried Schlenk tube, add the raw materials indoline (0.15 mmol, 30.4 mg), p-toluenesulfonamide (0.3 mmol, 51.1 mg), iodobenzene acetate (0.15 mmol, 48.3 mg), pentamethyl Cyclopentadienyl rhodium dichloride (5 mmol%, 4.6 mg), silver trifluoromethanesulfonate (20 mmol%, 7.7 mg), silver acetate (20 mmol%, 5.1 mg), acetic acid (0.45 mmol, 27 mg) 1.5 mL of 1,2-dichloroethane, and put the above-mentioned Schlenk tube at 100 o In a C oil bath, stir for about 16 h. The reaction was terminated, and the reaction solution was easily quenched with 2 mL of saturated ammonium chloride, and extracted several times with ethyl acetate (4 mL × 5), the organic phases were combined, and the solvent was removed on a rotary evaporator. Finally, after separation by silica gel column chromatography (eluent: et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com