Preparation method for P<1>, P<4>-di(uridine5'-)tetraphosphate
A technology of tetraphosphate and uridine is applied in the field of preparation of P1,P4-ditetraphosphate, and can solve the problems such as the method that cannot be used in practice and the low yield of target product.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
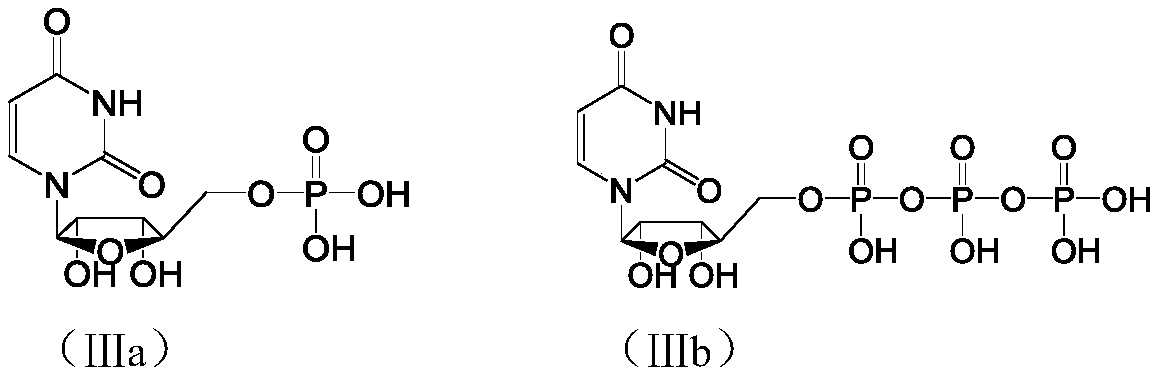
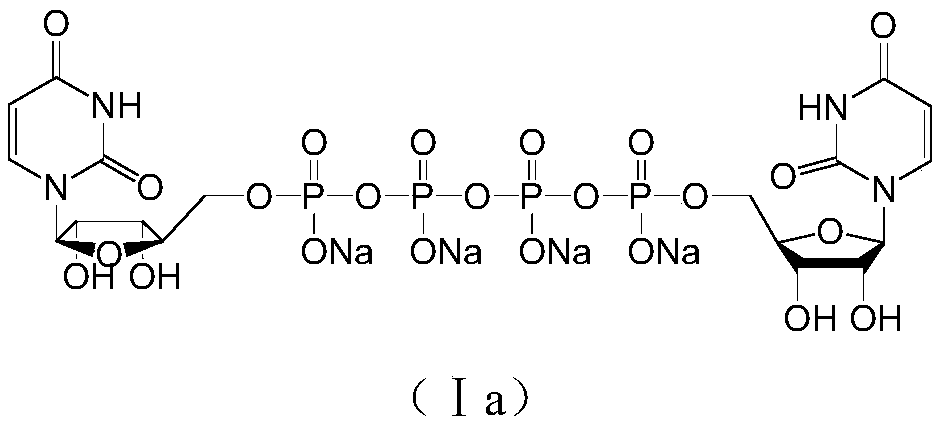
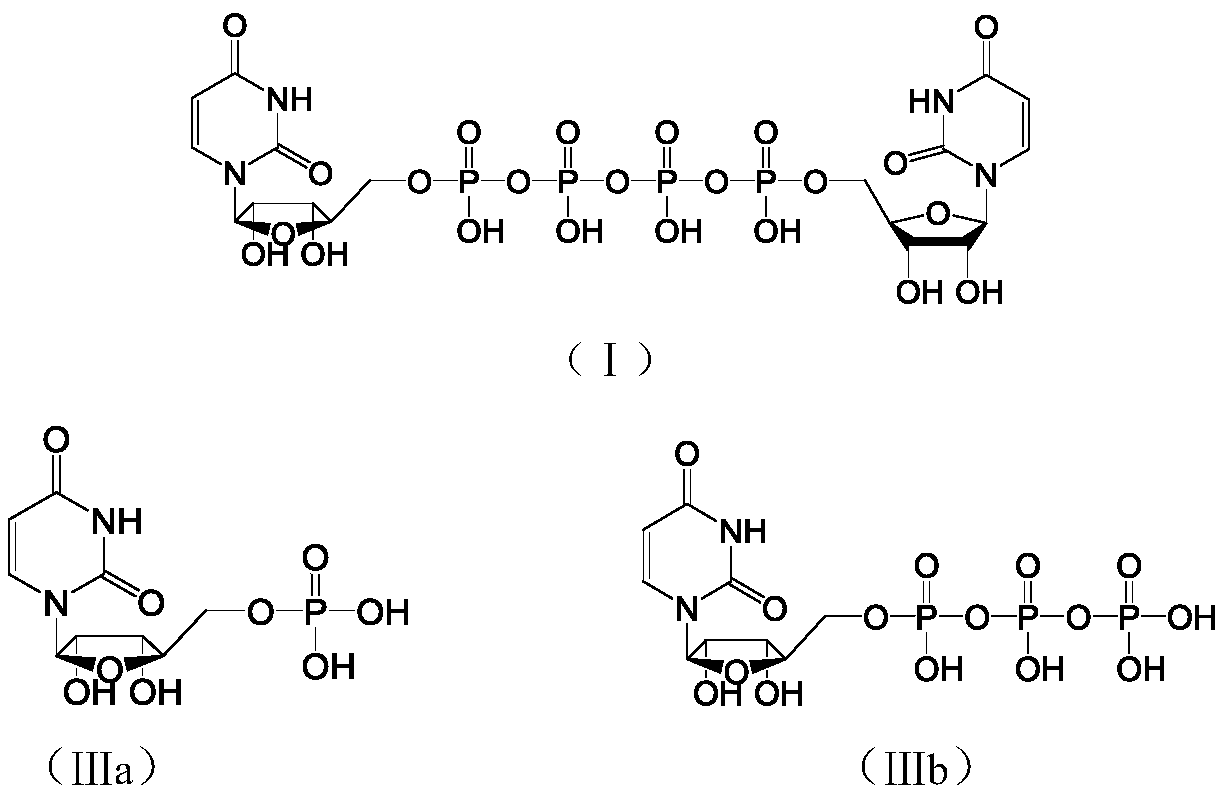
[0029] Example 1 P 1 ,P 4 - Preparation of two (uridine 5'-) tetraphosphate
[0030] The preparation (UMP-TBA) of the compound shown in step 1. intermediate formula (IIa)
[0031] Add uridine-5'-monophosphate disodium salt (5.43mol, 2.0kg) and purified water (10.0kg) into a 20L plastic bucket, dissolve completely under stirring and filter, and the filtrate is set aside; convert 20.0L into hydrogen form The DOWEX IR100S resin was loaded into the chromatography column, rinsed with purified water (40.0kg), compacted the resin, exhausted the washing liquid after discharging air bubbles, and turned off the drain switch; slowly introduced the above filtrate into the column to make the upper layer liquid The surface is just submerged in the resin, and after standing still, add purified water (95.0kg) to the column for elution. When the pH of the eluent becomes 6.5-7.5, stop the elution, combine the eluent, and add it to a 200L reaction kettle , started stirring, and added tri-n-bu...
Embodiment 2
[0036] Example 2P 1 ,P 4 - Preparation of two (uridine 5'-) tetraphosphate
[0037] Take the prepared N,N-dimethylformamide solution of UTP-3TBA (UTP-3TBA 3.84g, 3.69mmol) and place it in a 100ml three-necked flask, add diisopropylcarbodiimide (564mg, 4.44mmol) , N 2 After 2 replacements, react at 25-30°C for 5 hours. Add UMP-TBA in N,N-dimethylformamide solution (UMP-TBA 2.23g, 4.43mmol) and anhydrous magnesium chloride (422mg, 4.43mmol) to the reaction solution, then react at 25-30°C for 5 hours, Sampling HPLC detection, area normalization method to calculate UP4U content and large molecular weight impurity P 1 , P 5 Di(uridine 5′-)pentaphosphate (UP5U) and P 1 , P 6 - Content of bis(uridine 5'-)hexaphosphate (UP6U).
[0038] result:
[0039]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com