Antibodies that specifically bind to human il-15 and uses thereof
A specific and antibody technology, applied in the direction of antibodies, anti-animal/human immunoglobulins, specific peptides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0144] Transgenic rat production, immunization and hybridoma production
[0145] 1.1 IL-15 protein and IL-15Rα protein
[0146] Human interleukin 15 (IL-15) was purchased (Sigma), or soluble IL-15 receptor alpha (IL-15Rα ) (SEQ ID NO:512) was produced in the mammalian HEK293F expression system.
[0147] 1.2 Production of transgenic rats
[0148] Transgenic rats were generated as described in PCT Publication No. WO 08 / 151081. Briefly, the meganuclease expression construct is integrated into the genome of the test animal. Expression of meganucleases in germ cells causes double-strand breaks in endogenous rat immunoglobulin genes. Mating of such transgenic rats produces offspring with mutated / inactivated endogenous rat immunoglobulin genes.
[0149] Transgenic rats are further modified to carry artificial immunoglobulin genes, which allow the rats to produce antibodies with fully human variable regions.
[0150] 1.3 Immunity
[0151] To generate fully human monoclona...
Embodiment 2
[0156] Hybridoma Screening
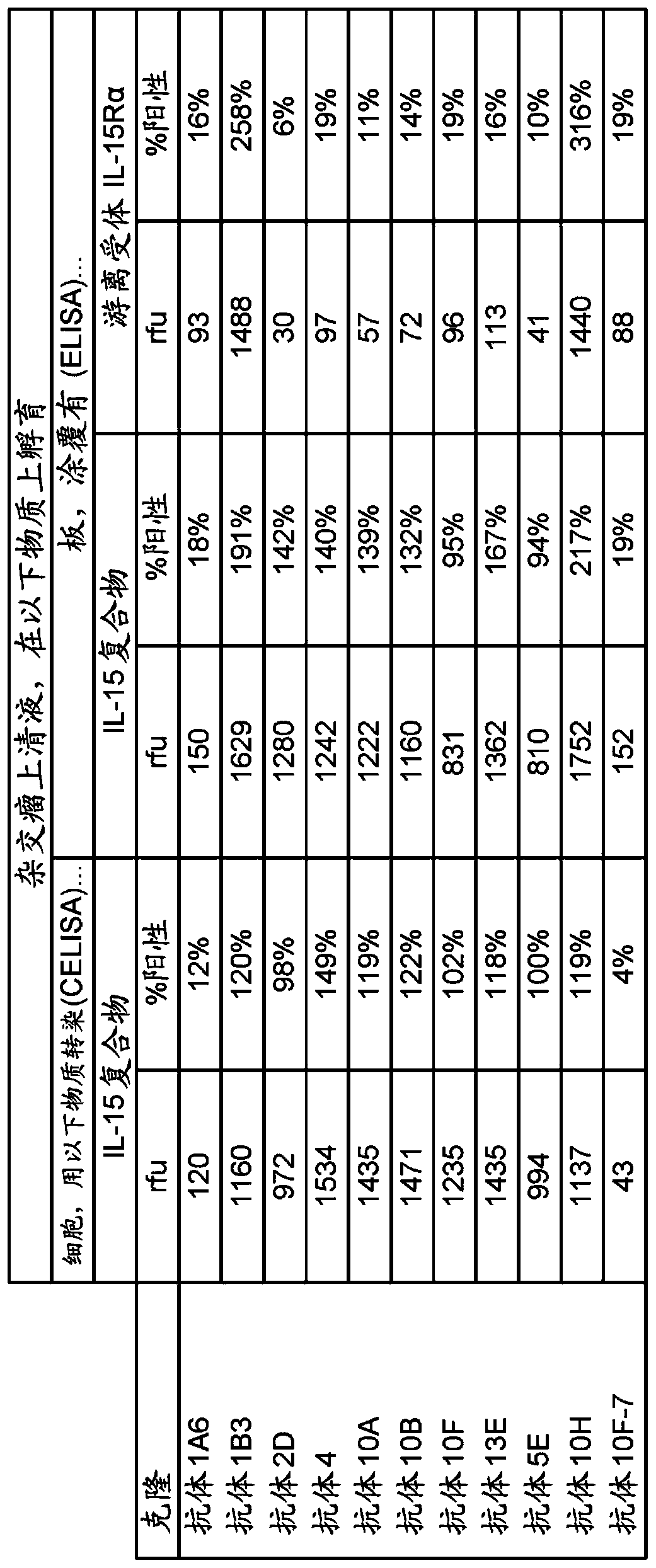
[0157] 2.1 Selection of antibodies that bind IL-15 complexed but not uncomplexed IL-15 receptor alpha using ELISA
[0158]Microtiter plates were coated with purified IL-15 or purified IL-15Rα or purified IL-15 complexes. Briefly, microtiter plates are coated with purified protein in PBS and then blocked with an unrelated protein such as bovine serum albumin (BSA) diluted in PBS. Dilutions of hybridoma supernatants were added to each well and incubated at 37°C for 1-2 hours. Use the board 20 washes followed by incubation for 1 hour at 37°C with goat anti-human IgG Fc-specific polyclonal reagent conjugated to a suitable detection reagent (eg horseradish peroxidase) alkaline phosphatase. After washing, the plate is developed with an appropriate substrate (eg 3,3',5,5'-tetramethylbenzidine TMD) and analyzed at an OD of 405. Hybridomas producing antibodies positively reactive with the IL-15 complex but not with IL-15Ra were selected for further c...
Embodiment 3
[0165] Identify antibody candidates for further development
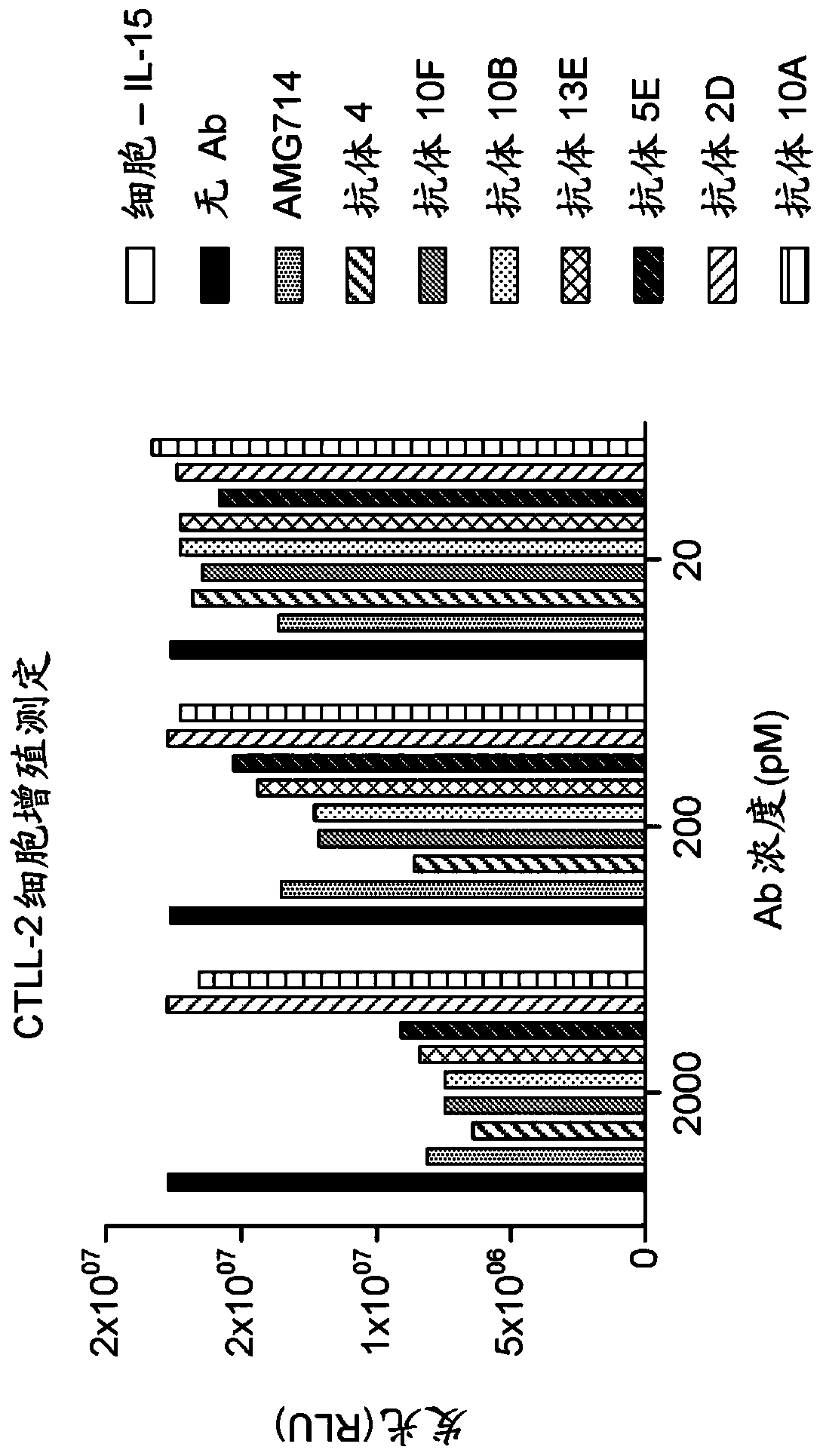
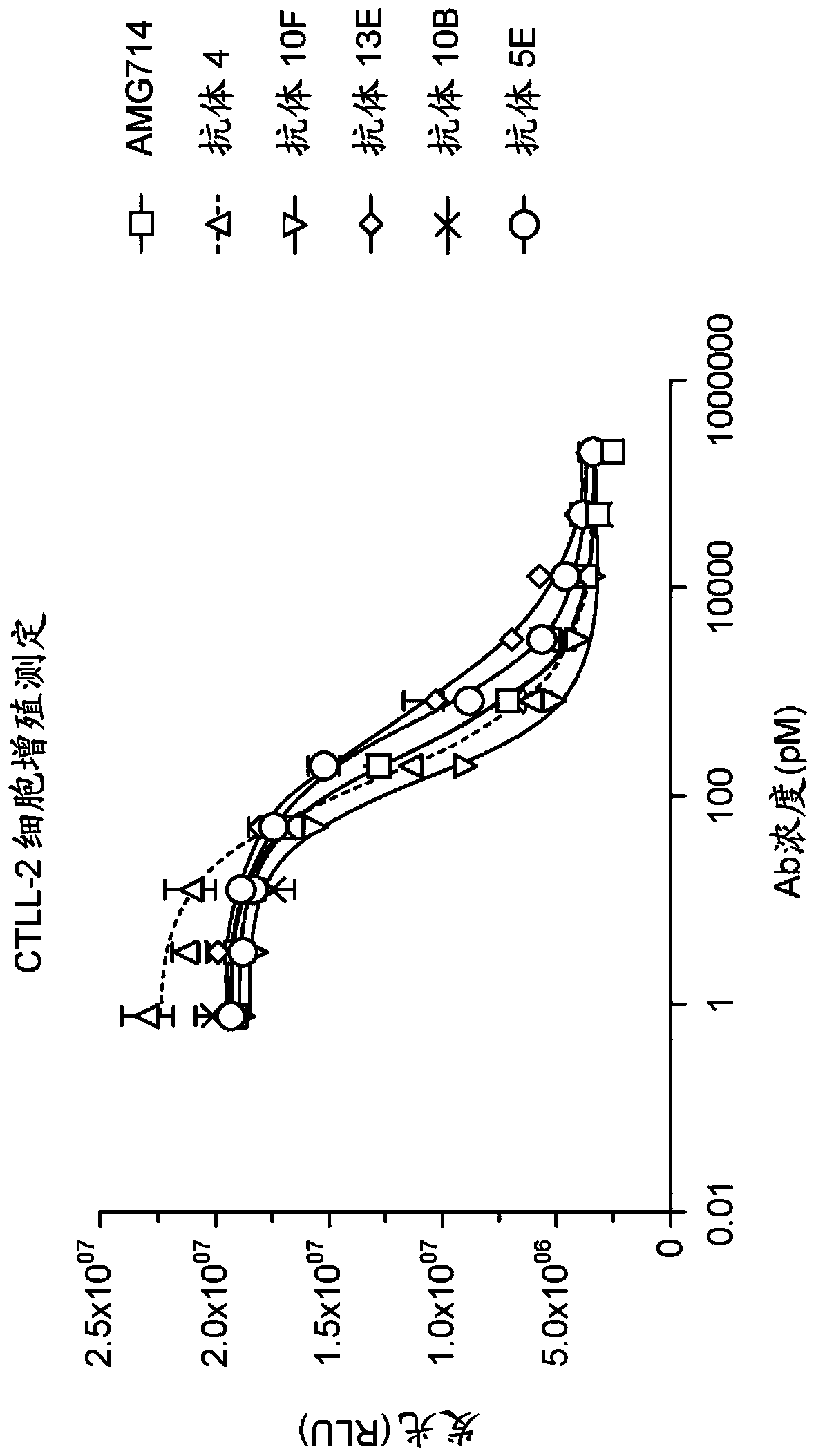
[0166] 3.1 Assay based on CTLL-2 cells
[0167] 1500 hybridoma samples that bound the IL-15 complex but not IL-15α were tested in a murine CTLL-2 cell-based assay to determine which neutralized the biological activity of IL-15. The CTLL-2 cell line is derived from cytotoxic T-cell lymphoma (ATCC: TIB-214) and responds to both IL-2 and IL-15.
[0168] Hybridoma supernatants (unpurified antibodies) were tested for their ability to neutralize IL-15-induced proliferation of CTLL-2 cells.
[0169] CTLL-2 cells were incubated in complete medium without IL-2 or IL-15 for 4 hours prior to testing. CTLL-2 cells (5x10 4 cells / well) were incubated with 200 pM IL-15 and IL-15 receptor α complex in a 96-well plate to induce cell proliferation. Hybridoma supernatants were added to the plates and incubated for 48 hours. Then use according to the manufacturer's instructions Inhibition of cell proliferation was assessed by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com