Alkyl-containing phthalonitrile monomer with autocatalytic curing effect, preparation method and application thereof
A technology of nitrophthalonitrile and phthalonitrile, which is applied in the field of phthalonitrile compounds and its preparation, can solve problems such as accelerating the curing of phthalonitrile resins, and achieve thermal stability and thermal oxidation Excellent stability and improved curing rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Preparation of monomer
[0032] Using 3.01g of bisphenol F (CAS No.: 620-92-8), 4-nitrophthalonitrile and potassium carbonate as raw materials, the molar ratio of bisphenol F to 4-nitrophthalonitrile is 1.0 : 2.3, the molar ratio of bisphenol F and potassium carbonate is 1.0:1.1;
[0033] Wherein the structure of bisphenol F is as follows:
[0034]
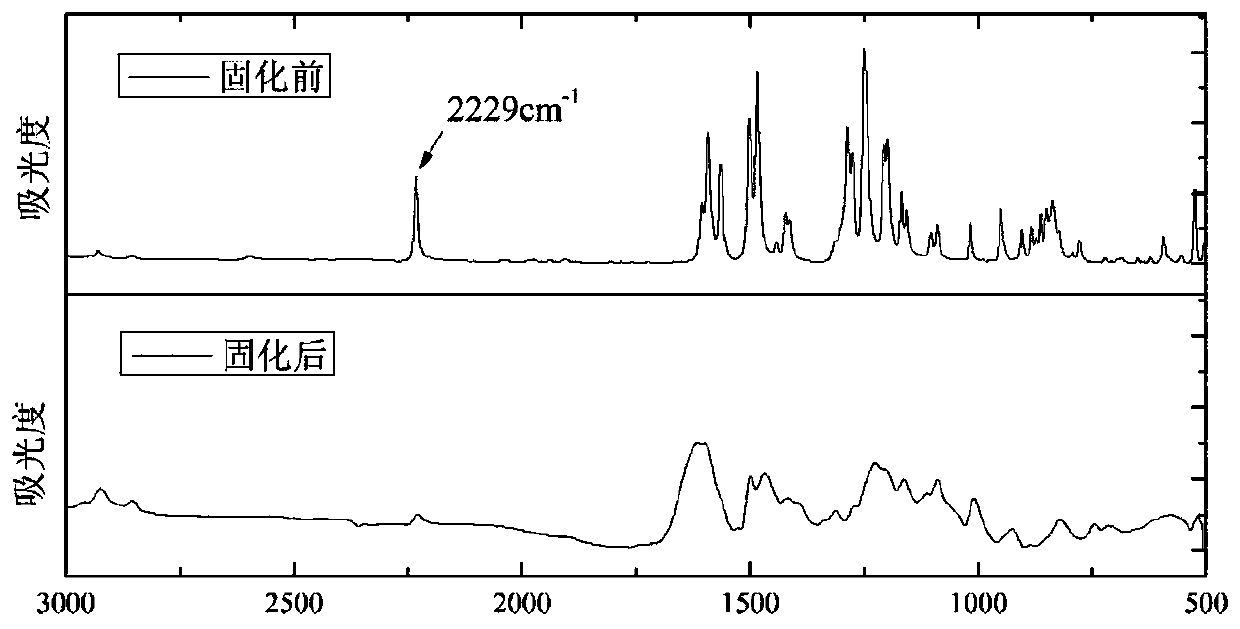
[0035] Dissolve bisphenol F and 4-nitrophthalonitrile together in 20 mL of NMP solvent, then add potassium carbonate to the solution, and keep the reaction at 30°C for 12 hours. After the reaction, the reaction liquid was washed twice with deionized water and methanol respectively and the filter cake was collected, and the filter cake was dried to constant weight to obtain the phthalonitrile monomer derived from bisphenol F. 1 The H-NMR structural formula is as follows:
[0036]
[0037] (2) Preparation of phthalonitrile resin
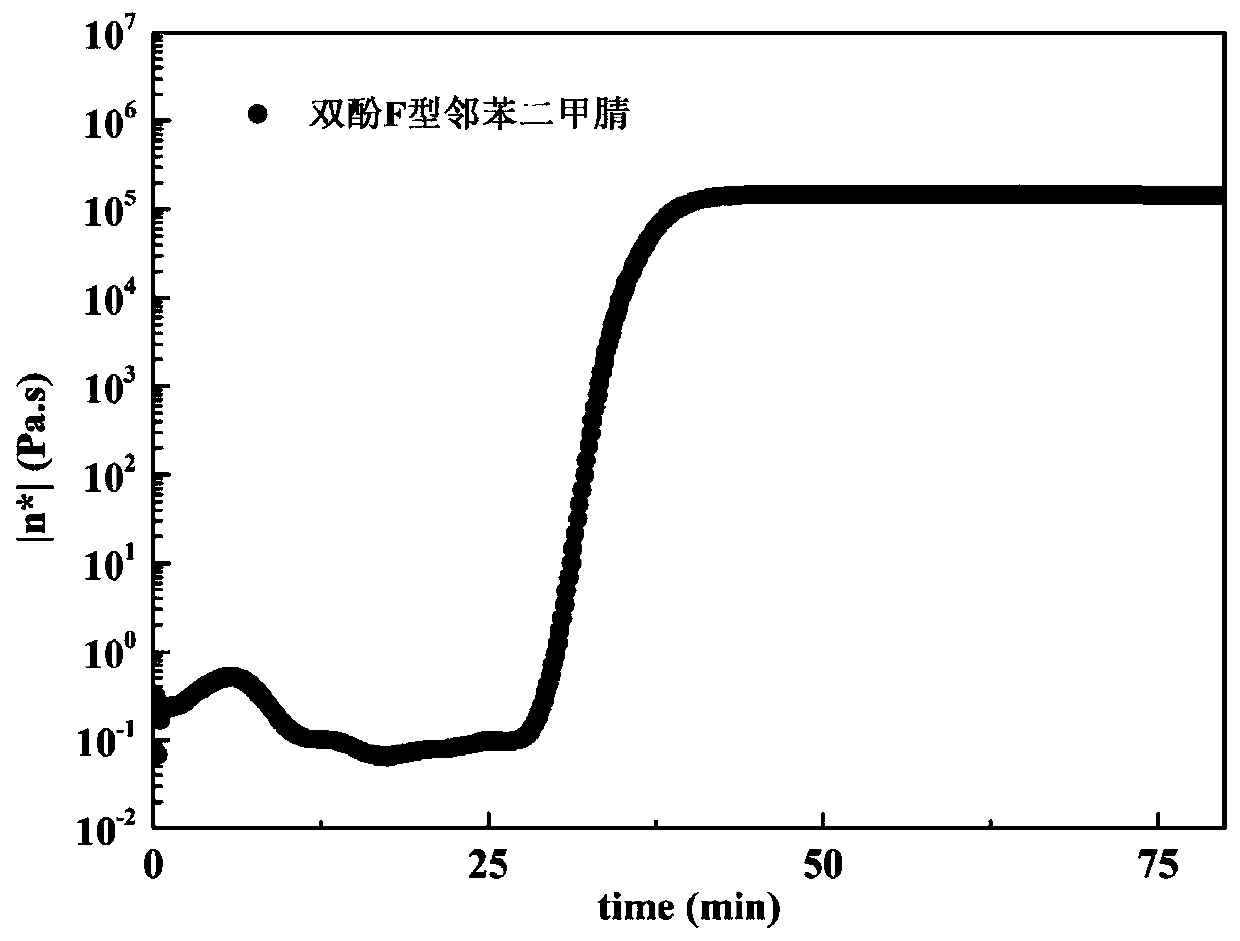
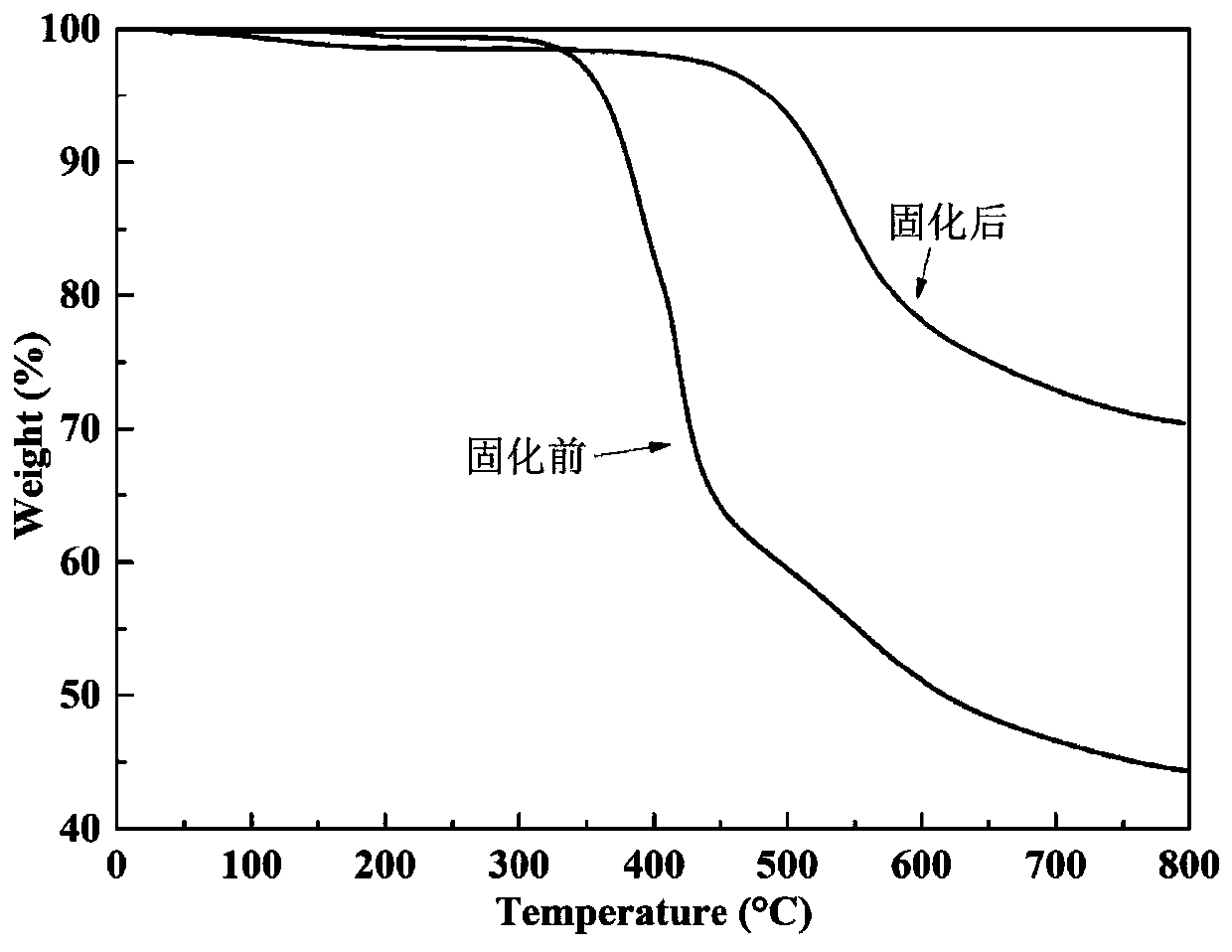
[0038] The alkyl-containing phthalonitrile monomer with self-catalyzed curing prop...
Embodiment 2
[0042] (1) Preparation of monomer
[0043] Using 3.2g of dihydric phenol (CAS No.: 2081-08-5), 4-nitrophthalonitrile and potassium carbonate as raw materials, the molar ratio of dihydric phenol to 4-nitrophthalonitrile is 1.0 : 2.3, the mol ratio of dihydric phenol and potassium carbonate is 1.0:1.1;
[0044] Wherein the structure of dihydric phenol is as follows:
[0045]
[0046] Dissolve dihydric phenol and 4-nitrophthalonitrile together in 25mL of NMP solvent, then add potassium carbonate to the solution, and keep the reaction at 30°C for 10 hours. After the reaction, the reaction solution was washed twice with deionized water and methanol respectively and the filter cake was collected, and the filter cake was dried to constant weight to obtain the corresponding dihydric phenol-derived phthalonitrile monomer, which 1 The H-NMR structural formula is as follows:
[0047]
[0048] (2) Preparation of phthalonitrile resin
[0049] The alkyl-containing phthalonitrile m...
Embodiment 3
[0053] (1) Preparation of monomer
[0054] Using 3.3g of dihydric phenol (CAS No.: 1576-13-2), 4-nitrophthalonitrile and potassium carbonate as raw materials, the molar ratio of dihydric phenol to 4-nitrophthalonitrile is 1.0 : 2.3, the mol ratio of dihydric phenol and potassium carbonate is 1.0:1.1;
[0055] Wherein the structure of dihydric phenol is as follows:
[0056]
[0057] Dissolve dihydric phenol and 4-nitrophthalonitrile together in 26 mL of DMSO solvent, then add potassium carbonate to the solution, and keep the reaction at 25°C for 15 hours. After the reaction, the reaction solution was washed twice with deionized water and methanol respectively and the filter cake was collected, and the filter cake was dried to constant weight to obtain the corresponding dihydric phenol-derived phthalonitrile monomer, which 1 The H-NMR structural formula is as follows:
[0058]
[0059] (2) Preparation of phthalonitrile resin
[0060] The alkyl-containing phthalonitrile...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Storage modulus | aaaaa | aaaaa |
| Storage modulus | aaaaa | aaaaa |
| Storage modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com