Method for improving in vitro maturation of oocytes in foaming stage after vitrification freezing
A technology of vitrification and oocytes, which is applied in the field of vitrification to improve the in vitro maturation of oocytes in the germinal and vesicular stage, to achieve the stable expression of genes related to the spindle assembly checkpoint, the increase of mitochondrial membrane potential and ATP levels, and the rate of abnormal morphology Falling effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
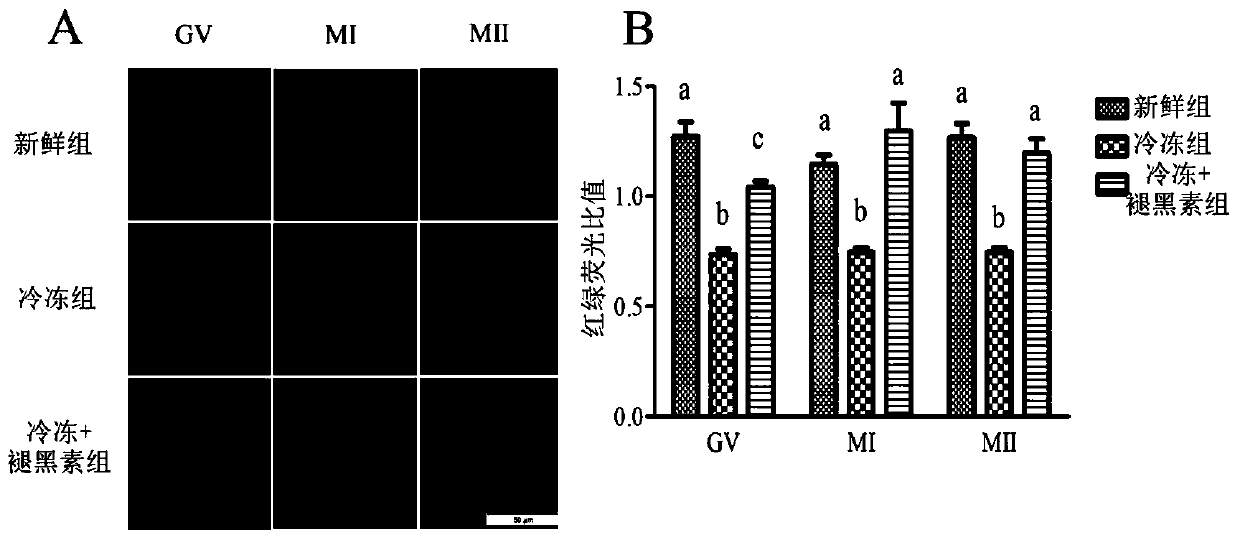
Image
Examples
Embodiment 1
[0028] This example provides a method for improving vitrification in vitro maturation of oocytes in germinal and vesicular stages, specifically through the following steps:
[0029] 1) Oocyte collection
[0030] 5-6 week-old female mice were raised at 18-22°C, with free access to food and water, and controlled light (14 hours / day). After two weeks of adaptive feeding, each mouse was injected with 10IU PMSG, and the ovary was collected 44-48 hours later, and the oocytes in the germinal vesicle stage with 2-3 layers of granulosa cells were collected and passed through M 2 Washed 3 times before use.
[0031] 2) Oocytes were vitrified, thawed and cultured in vitro for 1 hour
[0032] The plastic thin tube (250mL; IMV, L’Aigle, France) was heated and softened, and manually drawn into a thin tube with an inner diameter of 0.10mm and an outer diameter of 0.15mm, and a razor blade was used to cut OPS about 3cm long from the thin end for later use.
[0033] Freezing of oocytes: adop...
Embodiment 2
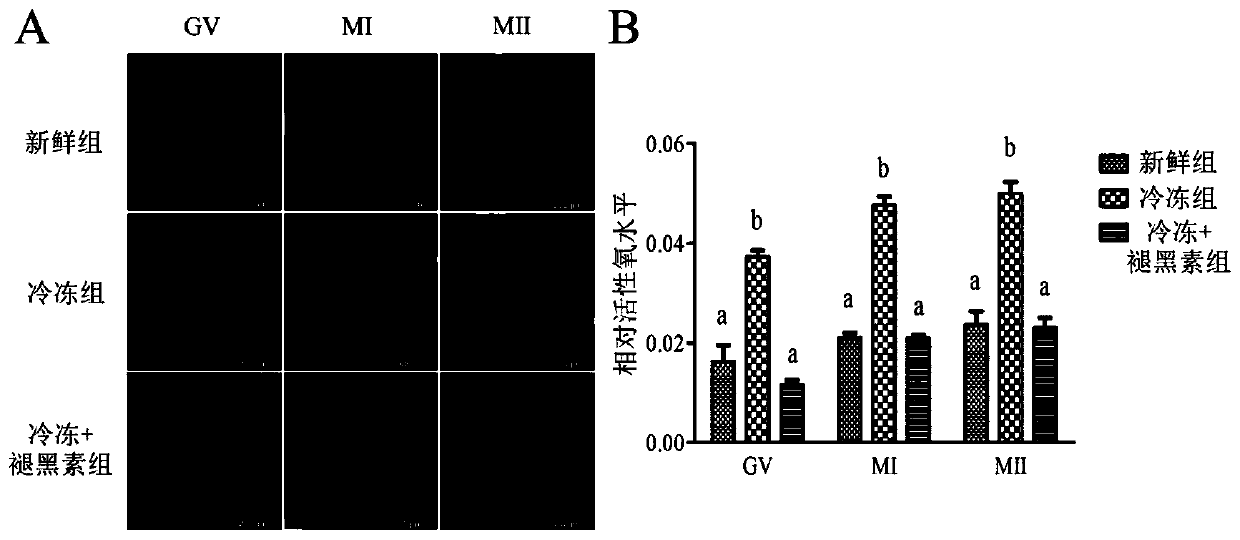
[0037] Example 2 Melatonin reduces the ROS level of mouse oocytes during in vitro maturation
[0038] After the frozen oocytes were thawed, the oocytes in different stages (GV, MI, and MII stages) of each group cultured in vitro for 0, 8, and 12 hours were tested for ROS levels. Oocytes in different stages (GV, MI and MII stages) of each group were washed three times in 1 mmol / LDCFH-DA (ROS staining solution) staining solution (DPBS as solvent), and then placed in 37.5 ° C, 5% CO 2 Stain in saturated humidity incubator for 30 minutes, after M 2 After washing three times with DAPI staining, the slides were stained with DAPI and observed under a fluorescent microscope (IX53 Olympus, Tokyo, Japan) after standing at 4°C in the dark for 2 hours. ROS detection was excited with blue light. The acquired image was saved in TIFF format, and the image was analyzed with ImageJ 1.48 to obtain the fluorescence intensity of each oocyte, and the background value was subtracted.
[0039] li...
Embodiment 3
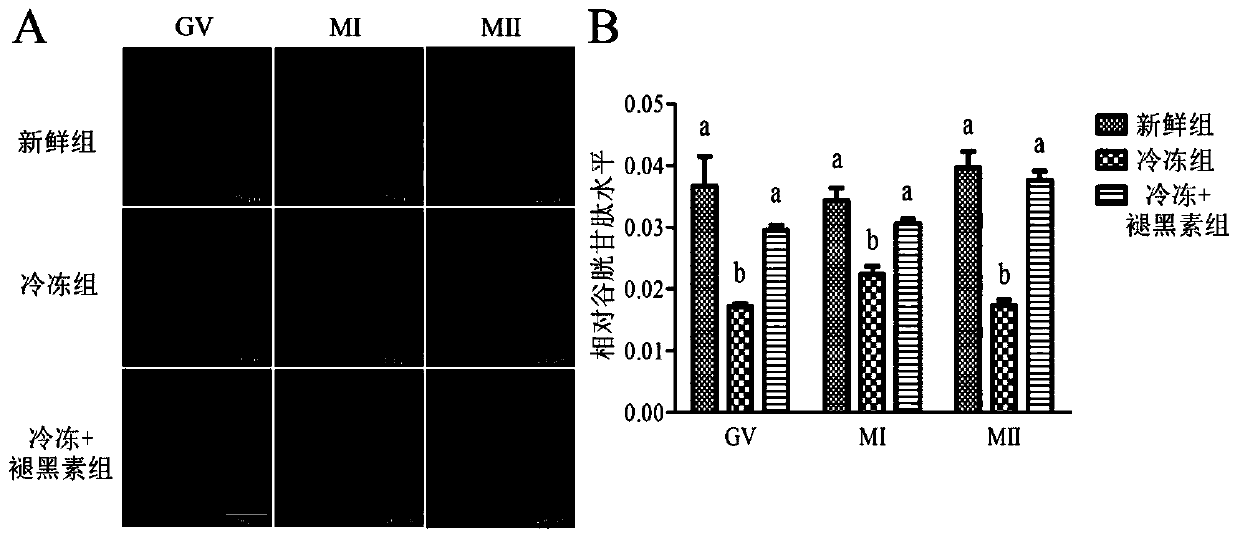
[0040] Example 3 Melatonin improves the GSH level of mouse oocytes during in vitro maturation
[0041] After the frozen oocytes were thawed, the oocytes in different stages (GV, MI and MII stages) of each group cultured in vitro for 0, 8, and 12 hours were subjected to GSH staining. Oocytes in different stages (GV, MI and MII stages) of each group were washed three times in 1 mmol / LDCFH-DA (ROS staining solution) staining solution (DPBS as solvent), and then placed in 37.5 ° C, 5% CO 2 Stain in saturated humidity incubator for 30 minutes, after M 2 After washing three times with DAPI staining, the slides were stained with DAPI and observed under a fluorescent microscope (IX53 Olympus, Tokyo, Japan) after standing at 4°C in the dark for 2 hours. GSH was stained with 10 μmol / L Cell Tracker Blue, the specific operation was as above, and excited with purple light.
[0042] It was found by GSH staining analysis ( figure 2 ), compared with the fresh group, the GSH levels of oocy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com