Method for detecting related substances in Loxoprofen or sodium salt thereof
A detection method and related substance technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems that the purpose of detection cannot be achieved, and whether it cannot be detected effectively, and achieve good peak shape, large income, and simple reagents Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
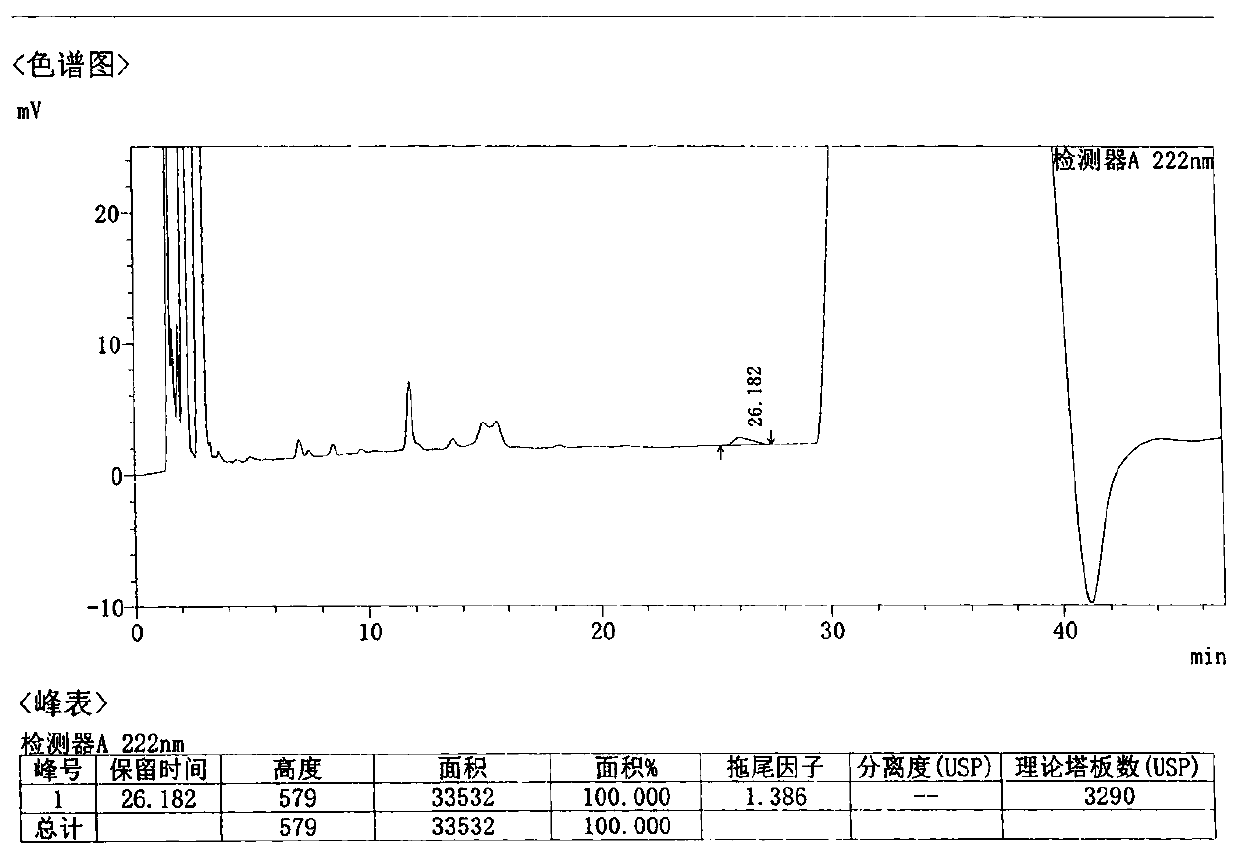
[0065] Take 1 loxoprofen sodium gel patch, cut the middle part 35cm 2 (equivalent to 25mg of loxoprofen sodium), cut into pieces into a 150ml conical flask, add 50ml of methanol, accurately weigh, sonicate for 30 minutes, let cool, add methanol to make up for the lost weight, filter, and take the subsequent filtrate , that is, the test solution is obtained. Precisely measure 10 μl of the test solution, inject it into a liquid chromatograph, measure and record the chromatogram.
[0066] The chromatographic conditions are as follows:
[0067] Column: Agilent Eclipse XDB-C18 4.6×150mm; 5μm
[0068] Mobile phase: 0.01mol / L sodium dihydrogen phosphate solution, add phosphoric acid to adjust pH to 2.5 and methanol gradient elution; detection wavelength: 222nm; flow rate: 1.0ml / min; column temperature: 35℃
[0069] The gradient elution procedure is as follows:
[0070] A: methanol;
[0071] B: 0.01mol / L sodium dihydrogen phosphate solution, add phosphoric acid to adjust pH to 2....
Embodiment 2
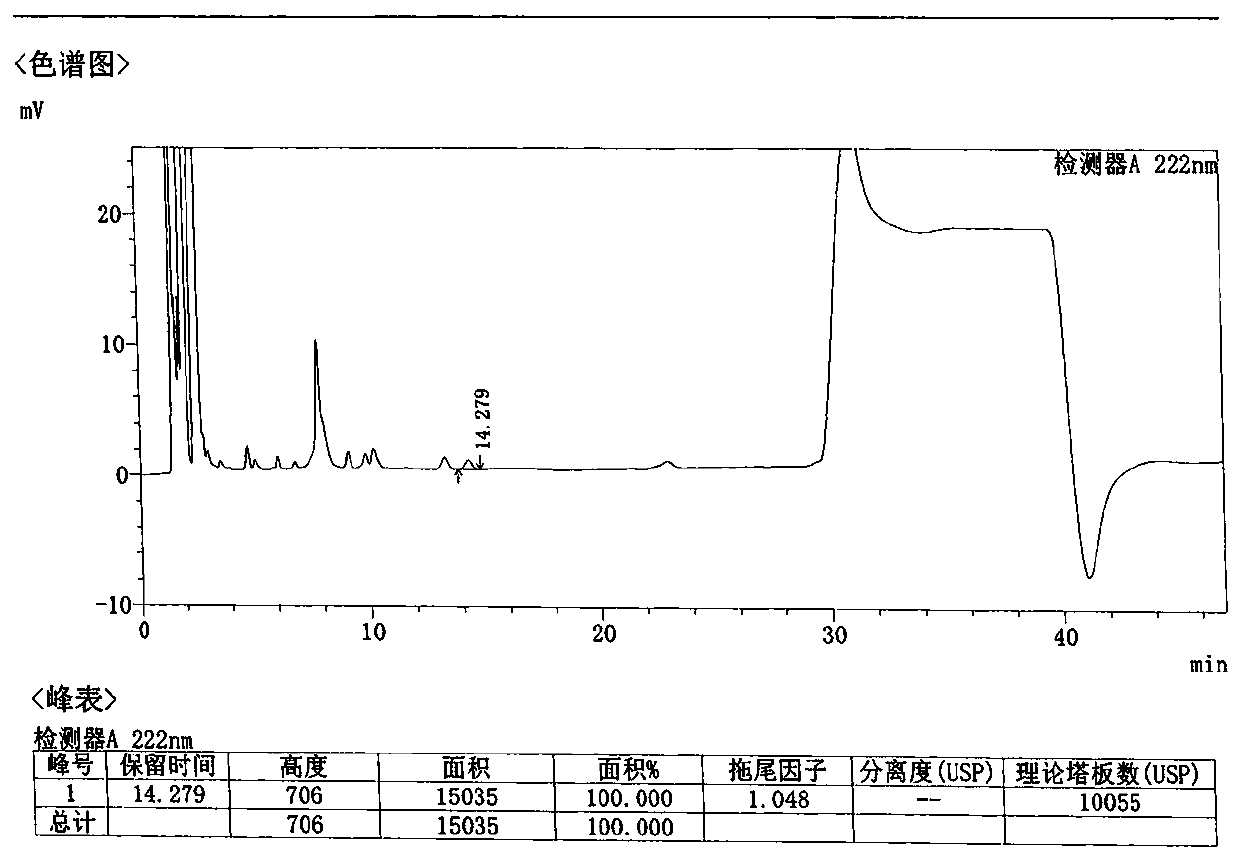
[0075] Take 1 loxoprofen sodium gel patch, cut the middle part 35cm 2 (equivalent to 25mg of loxoprofen sodium), cut into pieces into a 150ml conical flask, add 50ml of methanol, accurately weigh, sonicate for 30 minutes, let cool, add methanol to make up for the lost weight, filter, and take the subsequent filtrate , that is, the test solution is obtained. Precisely measure 10 μl of the test solution, inject it into a liquid chromatograph, measure and record the chromatogram.
[0076] The chromatographic conditions are as follows:
[0077] Column: Agilent Eclipse XDB-C18 4.6×150mm; 5μm
[0078] Mobile phase: 0.01mol / L sodium dihydrogen phosphate solution, add phosphoric acid to adjust pH to 2.5 and methanol gradient elution; detection wavelength: 222nm; flow rate: 1.0ml / min; column temperature: 35℃
[0079] The gradient elution procedure is as follows:
[0080] A: methanol;
[0081] B: 0.01mol / L sodium dihydrogen phosphate solution, add phosphoric acid to adjust pH to 2....
Embodiment 3
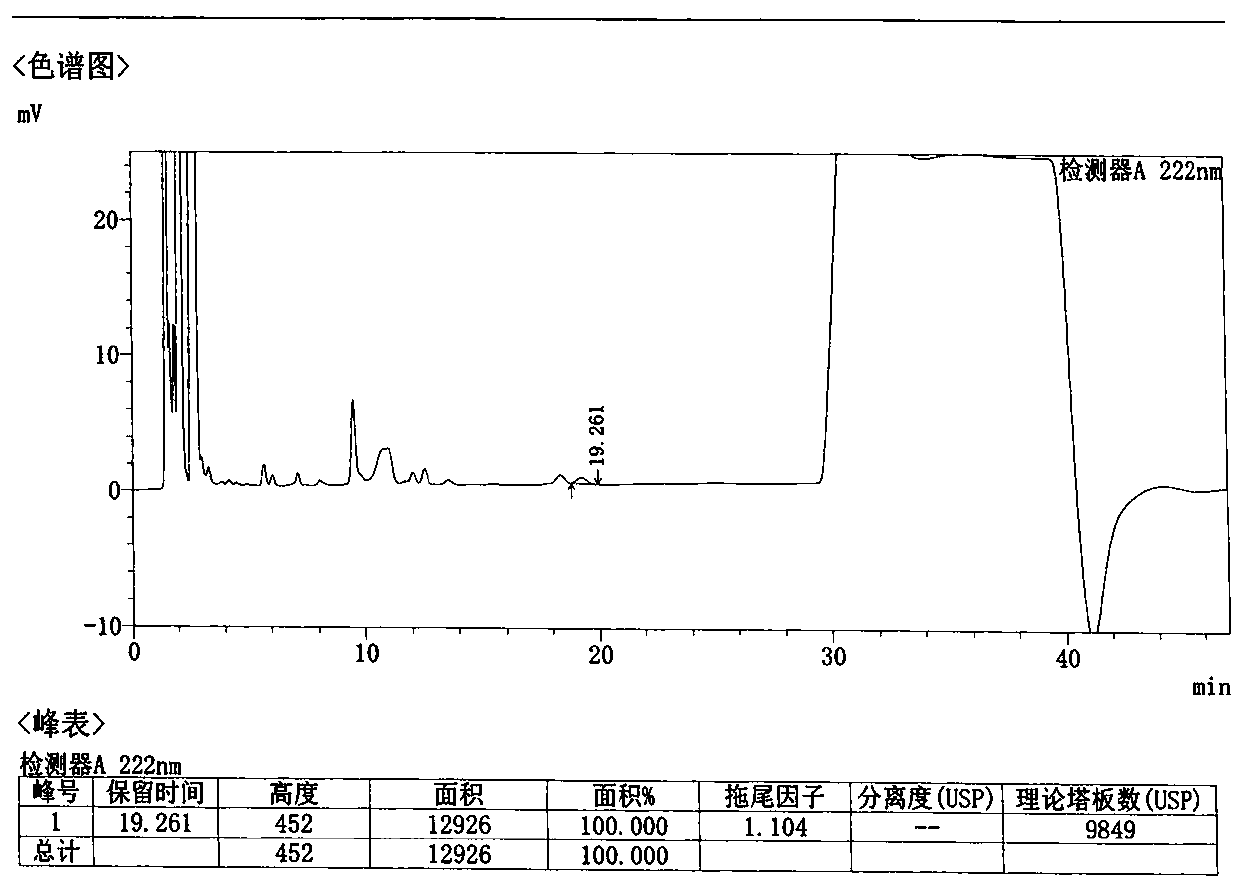
[0085] Take 1 loxoprofen sodium gel patch, cut the middle part 35cm 2 (equivalent to 25mg of loxoprofen sodium), cut into pieces into a 150ml conical flask, add 50ml of methanol, accurately weigh, sonicate for 30 minutes, let cool, add methanol to make up for the lost weight, filter, and take the subsequent filtrate , that is, the test solution is obtained. Precisely measure 10 μl of the test solution, inject it into a liquid chromatograph, measure and record the chromatogram.
[0086] The chromatographic conditions are as follows:
[0087] Column: Agilent Eclipse XDB-C18 4.6×150mm; 5μm
[0088] Mobile phase: 0.01mol / L sodium dihydrogen phosphate solution, add phosphoric acid to adjust pH to 2.5 and methanol gradient elution; detection wavelength: 222nm; flow rate: 1.0ml / min; column temperature: 35℃
[0089] The gradient elution procedure is as follows:
[0090] A: methanol;
[0091] B: 0.01mol / L sodium dihydrogen phosphate solution, add phosphoric acid to adjust pH to 2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com