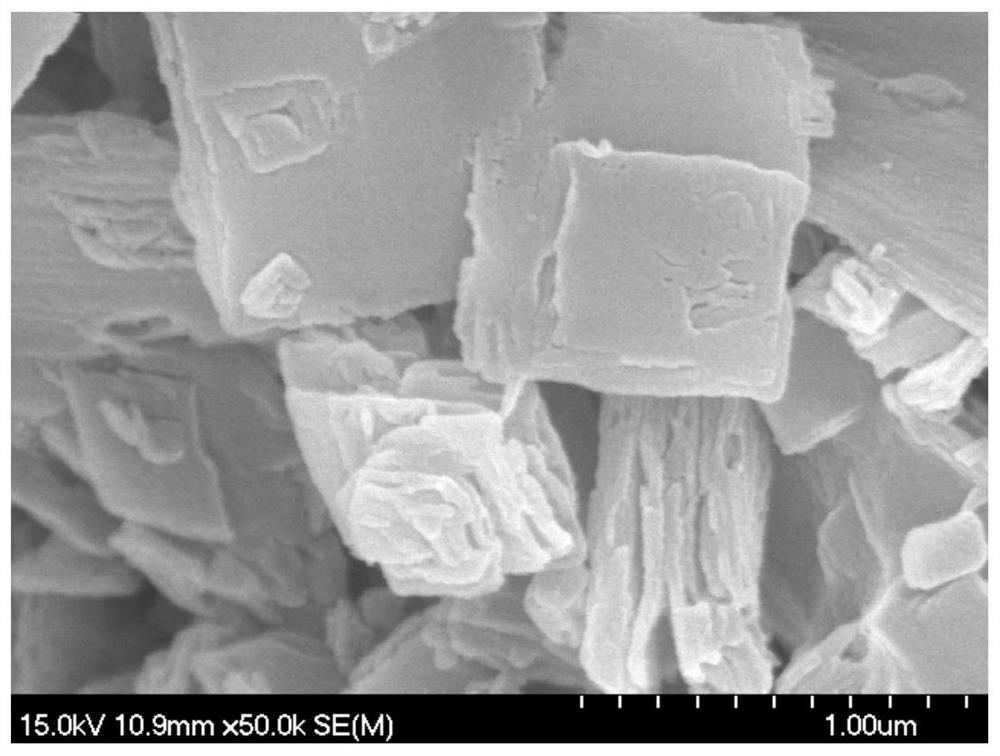
A kind of bimetallic organic framework nanoflower and its derivatives as well as preparation method and application
An organic framework and nanoflower technology, applied in organic compound/hydride/coordination complex catalysts, metal/metal oxide/metal hydroxide catalysts, separation methods, etc., can solve the problem of long reaction period and low reaction efficiency and other problems, to achieve a simple and gentle effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of ZIF-67@CoCuBDC nanoflowers and their derivatives:
[0034] First, 0.4832g of copper nitrate trihydrate was dissolved in 14ml of DMF, and 0.3323g of terephthalic acid was dissolved in 14ml of DMF. After ultrasonication was uniform, the mixture was sonicated for 30min in an ice bath, and then placed in a water bath at 80°C for 3 days. After the reaction is completed, the obtained reaction solution A is centrifuged, washed with DMF, and then washed with methanol until the washing solution is colorless, and dried to obtain CuBDC in blue powder. Then, weigh 0.2 g of activated CuBDC, dissolve it in 10 ml of ethanol to obtain an ethanol solution of CuBDC, weigh 1.6605 g of cobalt acetate tetrahydrate, and dissolve it in 25 ml of ethanol to obtain an ethanol solution of cobalt acetate tetrahydrate; weigh 1.642 g of 2 - methylimidazole is dissolved in 25ml of ethanol to obtain the ethanolic solution of 2-methylimidazole, then the ethanolic solution of the CuBD...
Embodiment 2
[0047] (3) Different solvents in CuBDC and cobalt acetate:
[0048] First, CuBDC was prepared according to the method of Example 1. Then, 0.2 g of the activated CuBDC was weighed and dissolved in 10 ml of DMF, methanol, DMAC, and DMSO, respectively. The subsequent steps were the same as those in Example 1.
[0049] ZIF-67@CoCuBDC obtained with DMF as solvent ZIF-67@CoCuBDC obtained for H 2 The flux is 8.5*10 -7 mol·s -1 m -2 ·Pa -1 , H 2 / CO 2 and H 2 / N 2 The selectivities of 8.5 and 12.1, respectively; the obtained Co 3 O 4 / CuO can completely oxidize CO at 115℃.
[0050] ZIF-67@CoCuBDC obtained with methanol as solvent for H 2 The flux is 2.1*10 -7 mol·s -1 m -2 ·Pa -1 , H 2 / CO 2 and H 2 / N 2 The selectivities are 6.5 and 8.1, respectively; the obtained Co 3 O 4 / CuO can completely oxidize CO at 125℃.
[0051] ZIF-67@CoCuBDC obtained with DMAC as solvent for H 2 The flux is 1.6*10 -7 mol·s -1 m -2 ·Pa -1 , H 2 / CO 2 and H 2 / N 2 The selectiv...
Embodiment 3
[0054] (4) Different reaction temperatures:
[0055] First, CuBDC was prepared according to the method of Example 1. Then, weigh 0.2 g of activated CuBDC, dissolve it in 10 ml of ethanol, weigh 1.6605 g of cobalt acetate tetrahydrate, add 25 ml of ethanol; It was put into the solution of CuBDC, mixed uniformly, and then transferred to a high-pressure reactor, and reacted at 100° C., 120° C., 180° C. and 200° C. for 2 days. After the reaction is completed, wash with DMF and ethanol for 2 to 3 times respectively.
[0056] The shapes of ZIF-67@CoCuBDC obtained at different reaction temperatures are different, and the CO catalytic effect is also different.
[0057] ZIF-67@CoCuBDC for H 2 The flux is 4.5*10 -8 mol·s -1 m -2 ·Pa -1 , H 2 / CO 2 and H 2 / N 2 The selectivities of 10.8 and 12.6, respectively; the obtained Co 3 O 4 / CuO can completely oxidize CO at 125℃.
[0058] Among them, the ZIF-67@CoCuBDC obtained at 120 °C has a 2 The flux is 3.6*10 -7 mol·s -1 m ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com