Deuterated imidazothiadiazole derivative and medical application thereof
A technology of thiadiazole derivatives and imidazolo, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, antineoplastic drugs, etc., can solve problems such as perishable, affecting metabolic stability, and reducing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
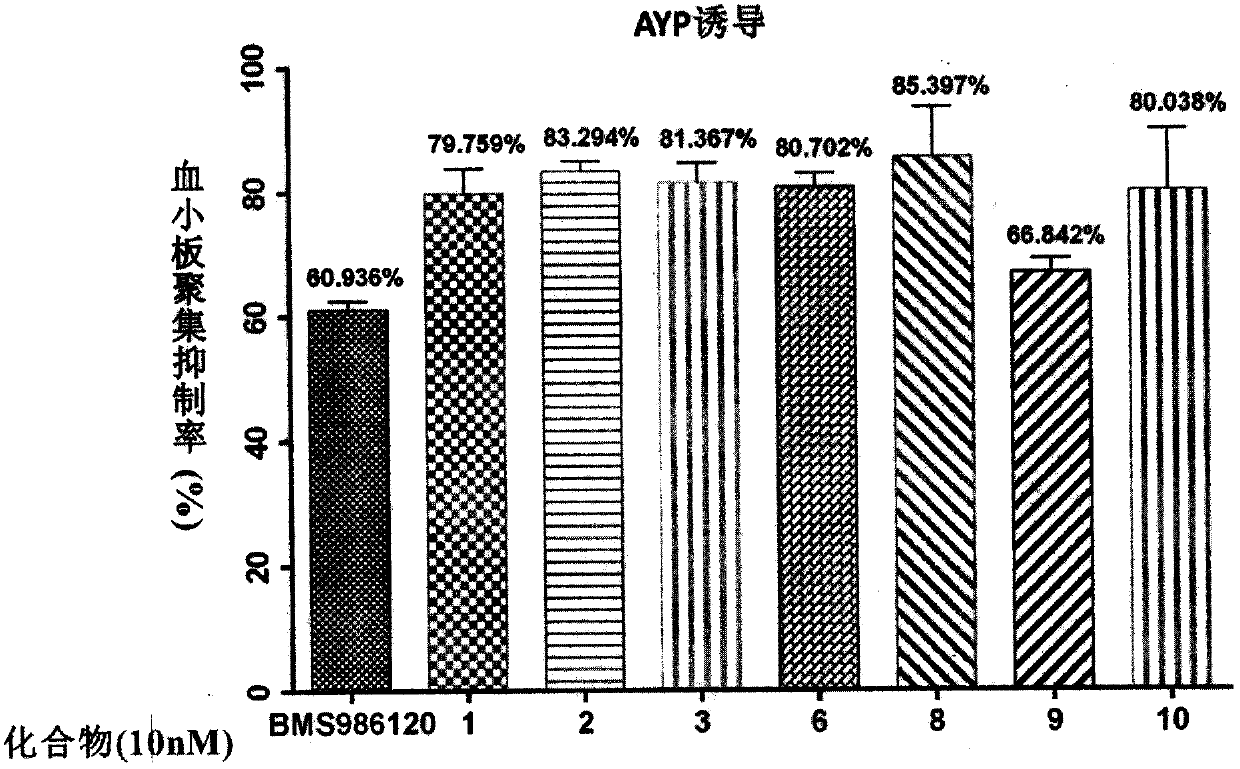
Image
Examples
Embodiment 1
[0094] 6-(4-(Benzyloxy)-6-(trideuteromethoxy)benzofuran-2-yl)-2-methoxyimidazo[2,1-b][1,3,4] Thiadiazole (compound 1)
[0095]
[0096] Under Ar atmosphere, phloroglucinol (3 g, 0.024 mol) was dissolved in anhydrous DMF (30 mL), and phosphorus oxychloride (2.2 mL, 0.024 mol) was slowly added dropwise at 0° C. Stir at room temperature for 4 hours. After the reaction, the reaction solution was poured into ice to quench, extracted with a mixed solvent (dichloromethane:methanol=10:1), concentrated, dissolved in ethyl acetate, and then repeatedly washed with saturated saline and 20% LiCl solution The remaining DMF was washed away, the solvent was evaporated to dryness under reduced pressure, and compound 1-1 (white solid, 2.9 g) was obtained by flash column chromatography (petroleum ether: ethyl acetate = 2:1), with a yield of 80%. 1 H NMR (300MHz, DMSO) δ11.46(s, 2H), 10.65(s, 1H), 9.93(s, 1H), 5.79(s, 2H).
[0097] Under the protection of Ar, 2,4,6-trihydroxybenzaldehyde (com...
Embodiment 2
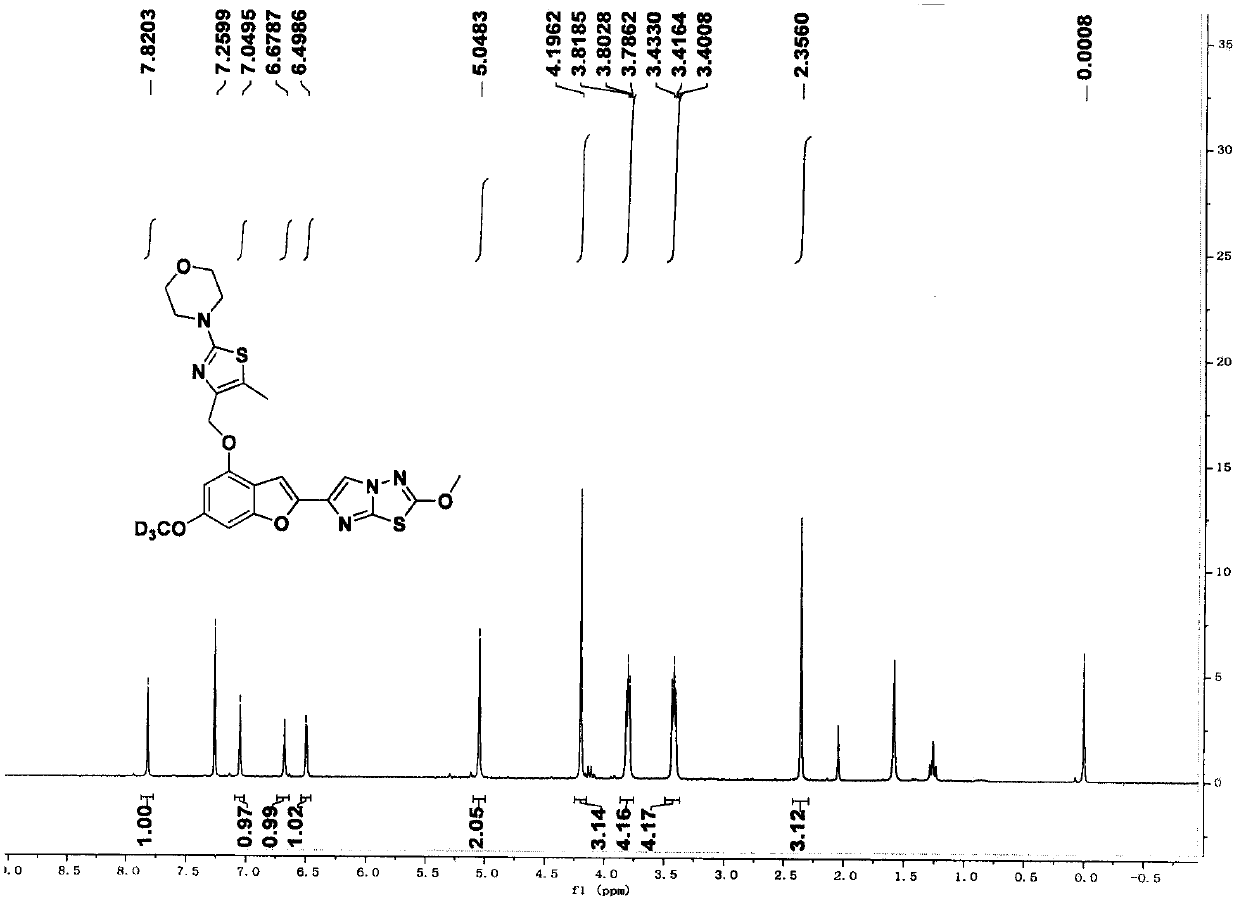
[0106] 4-(4-(((6-(trideuteromethoxy)-2-(2-methoxyimidazo[2,1-b][1,3,4]thiadiazol-6-yl) Benzofuran-4-yl)oxy)methyl)-5-methylthiazol-2-yl)morpholine (compound 2)
[0107]
[0108] The synthesis method of compound 2-1 refers to patent WO 2013 / 163279 A1.
[0109] (5-Methyl-2-morpholinothiazol-4-yl)methanol (compound 2-1) (0.25g, 1.16mmol) was dissolved in 5ml of anhydrous dichloromethane, and tribromo Phosphate (60 μL, 0.58 mmol) was dissolved in 1 ml of dichloromethane, and was added dropwise to the above system. After the addition was complete, react at room temperature for 4 hours, and add 10 ml of saturated sodium bicarbonate solution to quench the reaction, and extract the aqueous phase with dichloromethane. (5mLx3), the organic phase was combined, the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, the organic phase was evaporated to dryness to obtain a crude product, and the compound 2- 2 (white crystal, 0.145g), yield 45%.
[0110]...
Embodiment 3
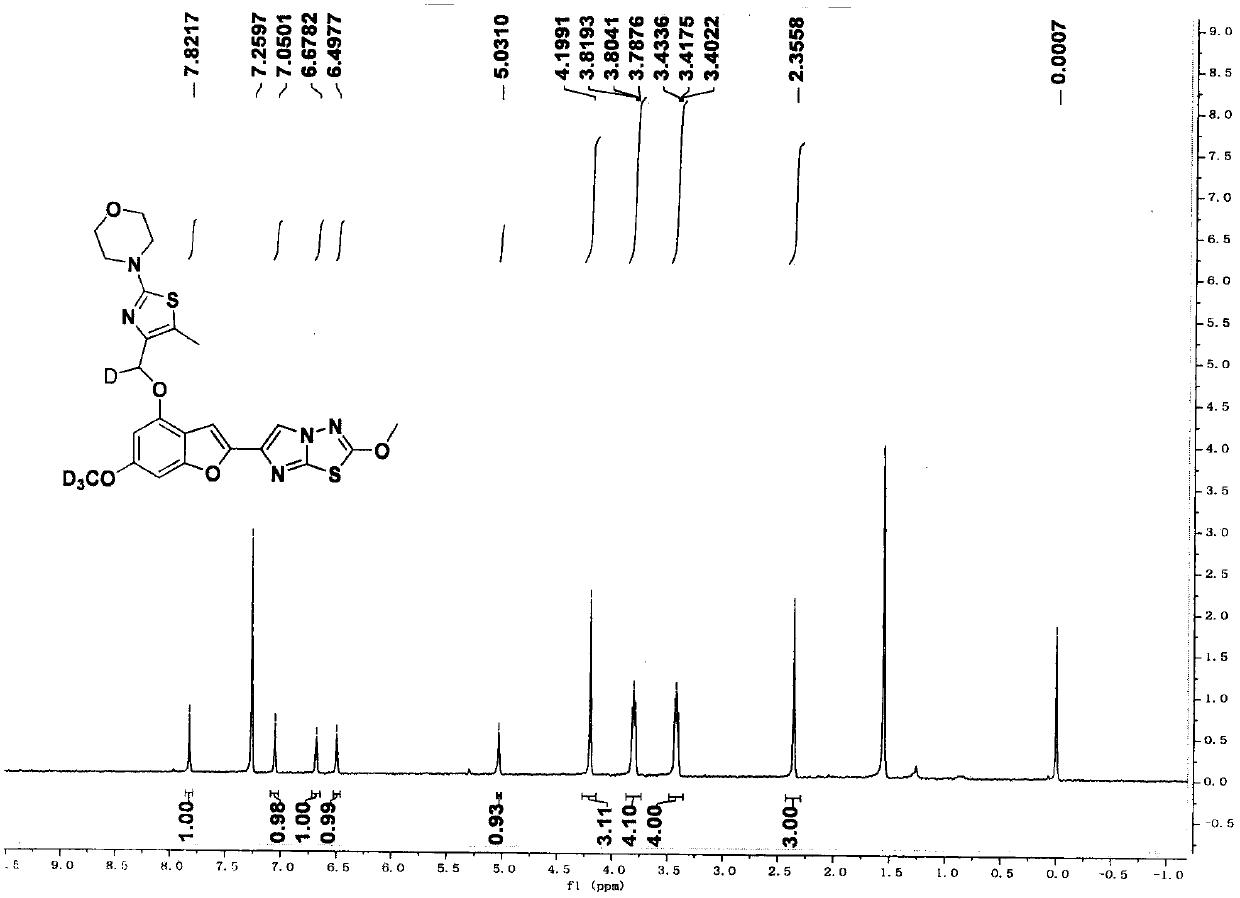
[0113] 4-(4-(((6-(trideuteromethoxy)-2-(2-methoxyimidazo[2,1-b][1,3,4]thiadiazol-6-yl) Benzofuran-4-yl)oxy)methyl-deuterium)-5-methylthiazol-2-yl)morpholine (compound 3)
[0114]
[0115] Dissolve (5-methyl-2-morpholinothiazol-4-yl)methanol (compound 2-1) (0.05g, 0.23mmol) in 5ml of dichloromethane solution, add pyridine (45μL, 0.56mmol), Dess Martin reagent (0.12g, 0.28mmol), reacted at room temperature for 2h, TLC monitored the reaction to be basically complete, filtered, washed the filter cake with dichloromethane, collected the filtrate, concentrated, flash column chromatography (petroleum ether: ethyl acetate = 4: 1) to obtain compound 3-1 (light cyan solid, 0.032g), yield: 65%.
[0116] 5-Methyl-2-morpholinothiazole-4-carbaldehyde (compound 3-1) (0.187g, 0.88mmol) was dissolved in 8ml of deuterated methanol, and sodium borodeuteride (0.037g, 0.88mmol), keep the reaction at room temperature for 5h, TLC monitoring after the completion of the reaction, add 5ml of water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com