Method and system for selecting individualized tumor neoantigen based on expected curative effect
An antigen and tumor technology, applied in biological systems, medical automated diagnosis, computer-aided medical procedures, etc., can solve the problems of unclear correlation mechanism, inability to find immunogenic tumor neoantigens, difficult tumor neoantigens, etc., to improve prediction The effect of precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
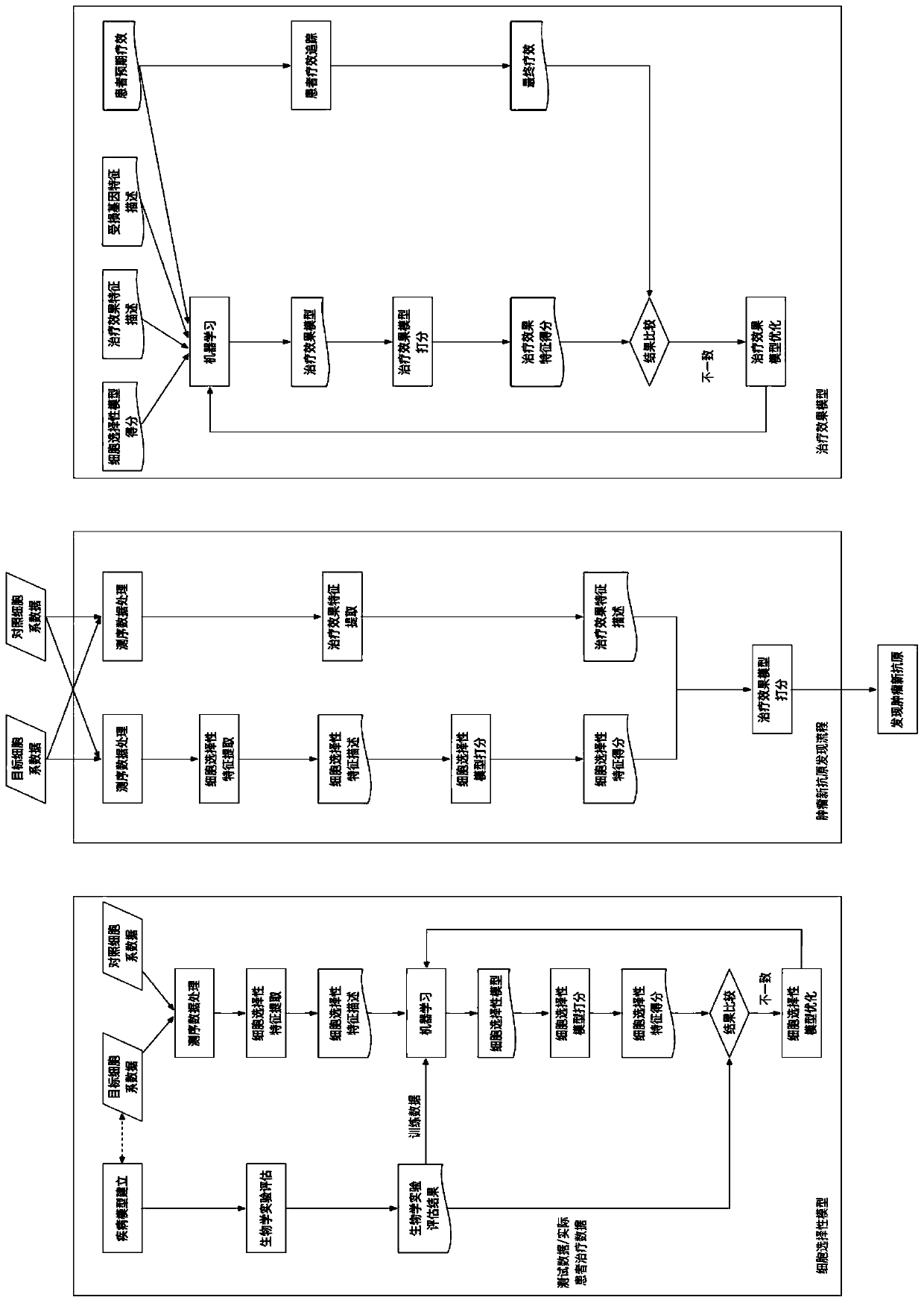
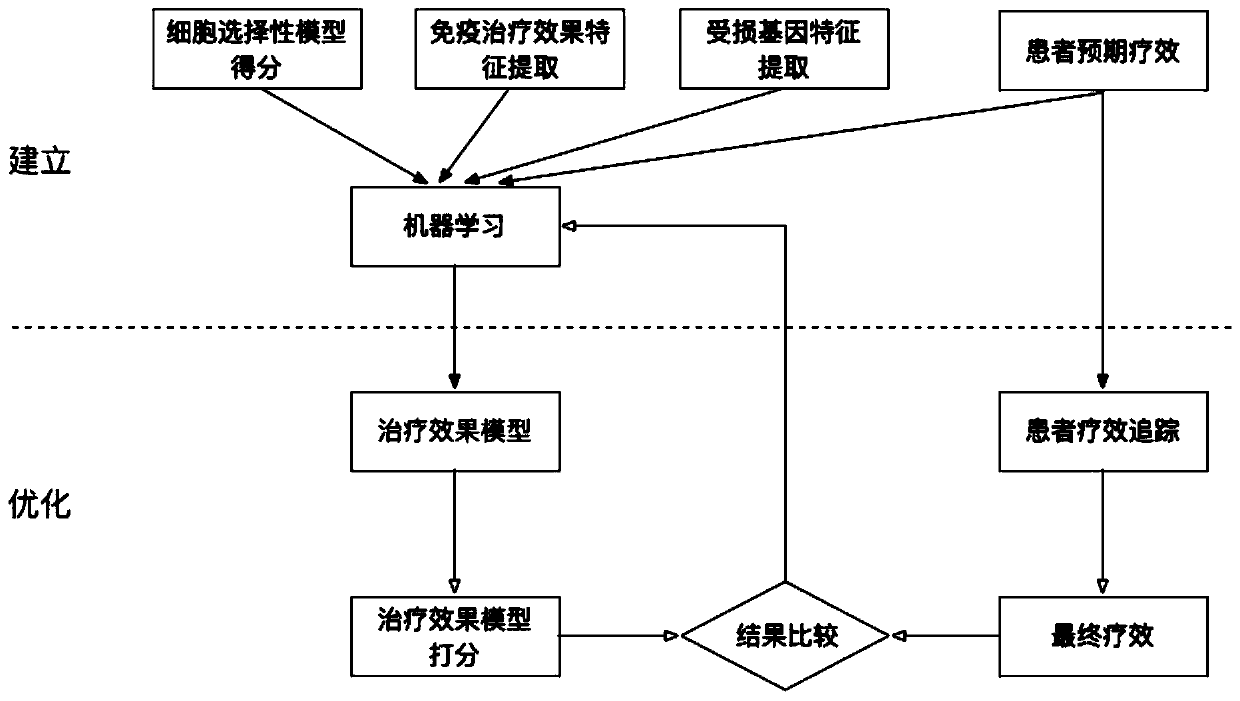
[0080] The present invention discloses a method and system for selecting individualized tumor neoantigens based on curative effect prediction. The specific method includes the following contents:
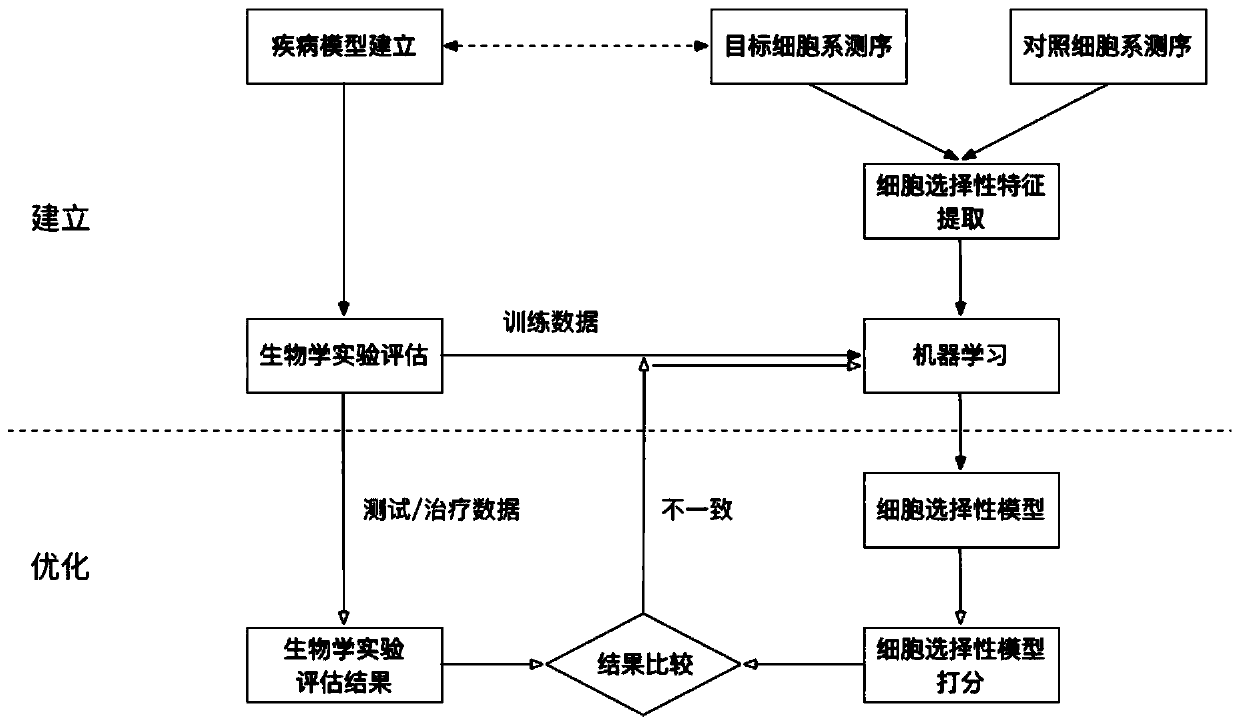
[0081] figure 2 Schematic diagram of the establishment and optimization of the cell selectivity model for the technical solution of the present invention:
[0082] 1. Establishment and optimization of cell selection model:
[0083] The cell-selective model is related to the selection ability of tumor cells, and the model is available immediately after establishment, and its accuracy is continuously optimized. Before the model is established, the method published in the literature is used to make a preliminary prediction of the cell selectivity of the neoantigen. The preliminary prediction results were formed into a theoretical training data set for model fitting, and an initial model with comparable prediction accuracy to the literature was obtained. After the model is establish...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com