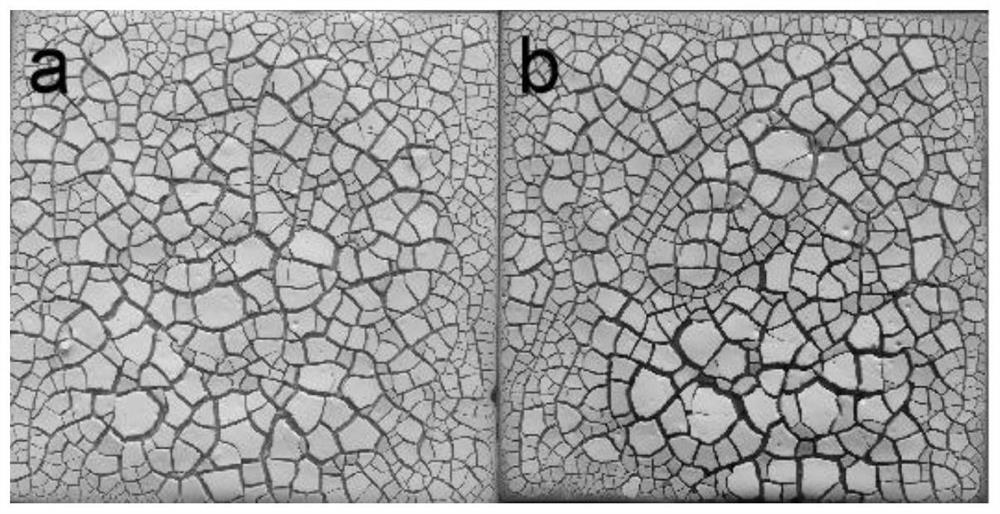
Preparation method of corrosive self-brittle radioactive detergent for iron-based material surface
A technology for iron-based materials and detergents, used in radioactive purification, nuclear engineering, etc., can solve the problems of poor acid and alkali corrosion resistance, large amount of secondary waste, self-brittle detergents corroding metal surfaces, etc. Acid resistance and less secondary waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
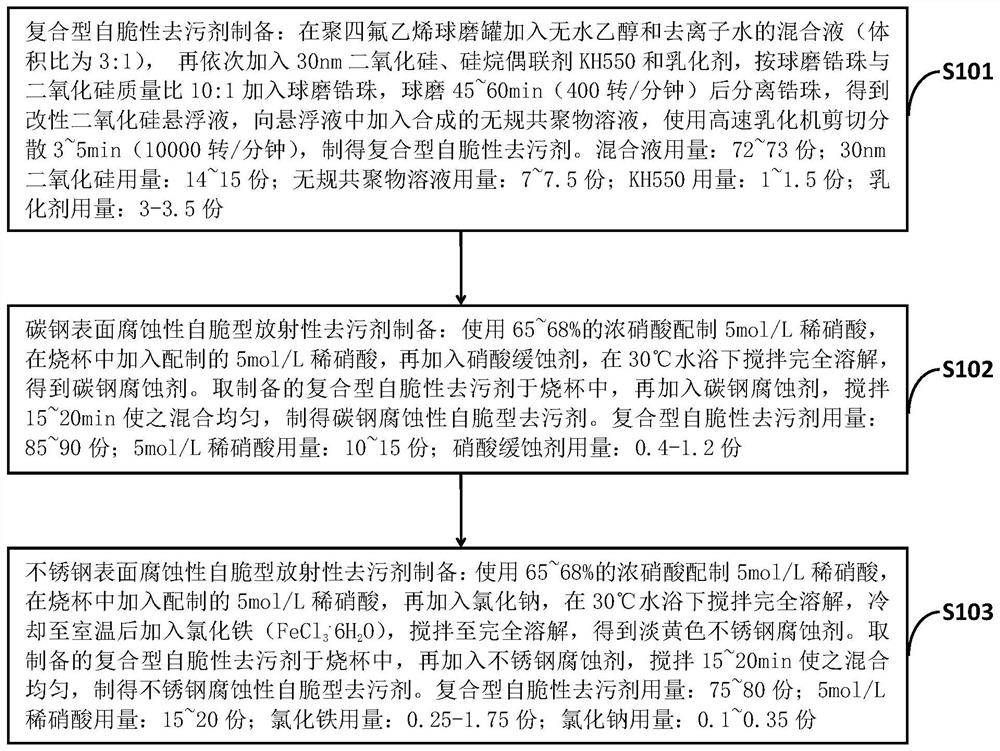
[0045] Such as figure 1 As shown, the preparation method of the corrosive self-brittle type radioactive detergent for the surface of iron-based materials according to the embodiment of the present invention, based on 100 parts of the synthetic compound self-brittle type radioactive detergent, comprises the following steps:
[0046] S101: Preparation method of composite self-brittle detergent: add a mixture of absolute ethanol and deionized water (volume ratio 3:1) into a polytetrafluoroethylene ball mill tank, then add 30nm silica, silane coupling Add agent KH550 and emulsifier, add ball-milled zirconium beads according to the mass ratio of ball-milled zirconium beads to silica 10:1, separate zirconium beads after ball-milling for 45-60min (400 rpm), and obtain a modified silica suspension. Add the synthesized random copolymer solution into the liquid, and use a high-speed emulsifier to shear and disperse for 3 to 5 minutes (10000 revolutions per minute) to obtain a composite ...
Embodiment 1
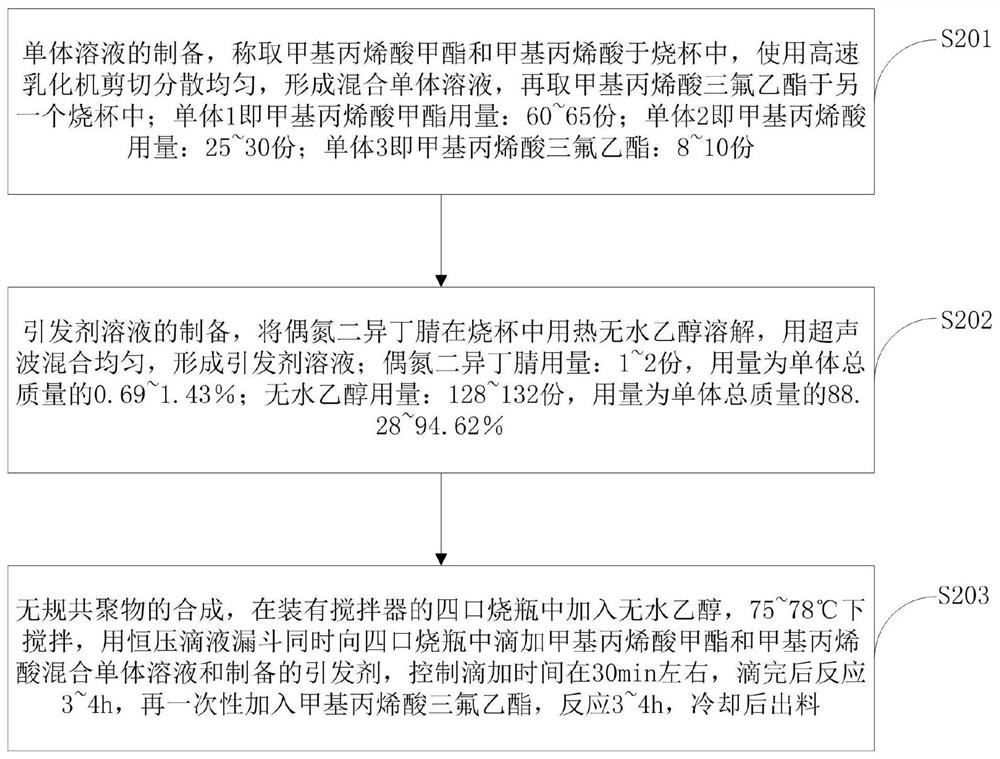
[0055] The resin synthesis that the embodiment of the present invention provides comprises:
[0056] 1) Preparation of monomer solution: Weigh 120.00 g of methyl methacrylate (MMA), 50.00 g of methacrylic acid (MAA), 15.90 g of trifluoroethyl methacrylate (TFEMA), and high-speed shear the MMA and MAA Mix evenly for later use, and place TFEMA in a beaker separately for later use.
[0057] 2) Preparation of initiator solution: Dissolve 1.95g of AIBN in 100mL of hot ethanol, and disperse by ultrasonic for later use.
[0058] 3) Synthesis of resin: Add 900mL of absolute ethanol to a 2000mL four-necked flask, stir in a water bath at 75°C, and simultaneously add methyl methacrylate and methacrylic acid to the four-necked flask dropwise with a constant pressure dropping funnel. Bulk solution and prepared initiator, control the dropping time at about 30 minutes, react for 3-4 hours after dropping, then add trifluoroethyl methacrylate at one time, react for 3-4 hours, and discharge af...
Embodiment 2
[0060] The preparation of the iron-based material surface corrosive self-brittle type radioactive detergent provided by the embodiments of the present invention includes:
[0061] 1) Preparation method of composite self-brittle detergent: add 100g of a mixture of absolute ethanol and deionized water (volume ratio: 3:1) to a polytetrafluoroethylene ball mill tank, then add 20g of 30nm silicon dioxide, 2g of Silane coupling agent KH550 and 5g emulsifier, add ball-milled zirconium beads according to the mass ratio of ball-milled zirconium beads to silica 10:1, separate zirconium beads after ball milling for 45-60min (400 rpm), and obtain modified silica suspension Add 10g of synthetic random copolymer solution to the suspension, and use a high-speed emulsifier to shear and disperse for 3 to 5 minutes (10,000 revolutions per minute) to obtain a composite self-brittle detergent.
[0062] 2) Preparation method of carbon steel surface corrosive self-brittle radioactive detergent: use...
PUM
| Property | Measurement | Unit |
|---|---|---|
| depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com