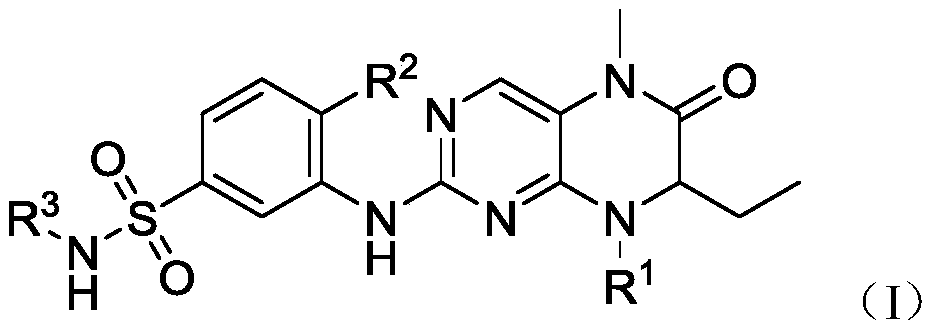
Dihydropteridinone-sulfamide derivatives, pharmaceutically-acceptable salts of derivatives, preparation method of derivatives and application of derivatives and salts
A technology of dihydropteridone and sulfonamide, which can be applied in the fields of organic chemistry, drug combination, anti-tumor drugs, etc., can solve the problem that there are not many BRD4 small molecule inhibitors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 29
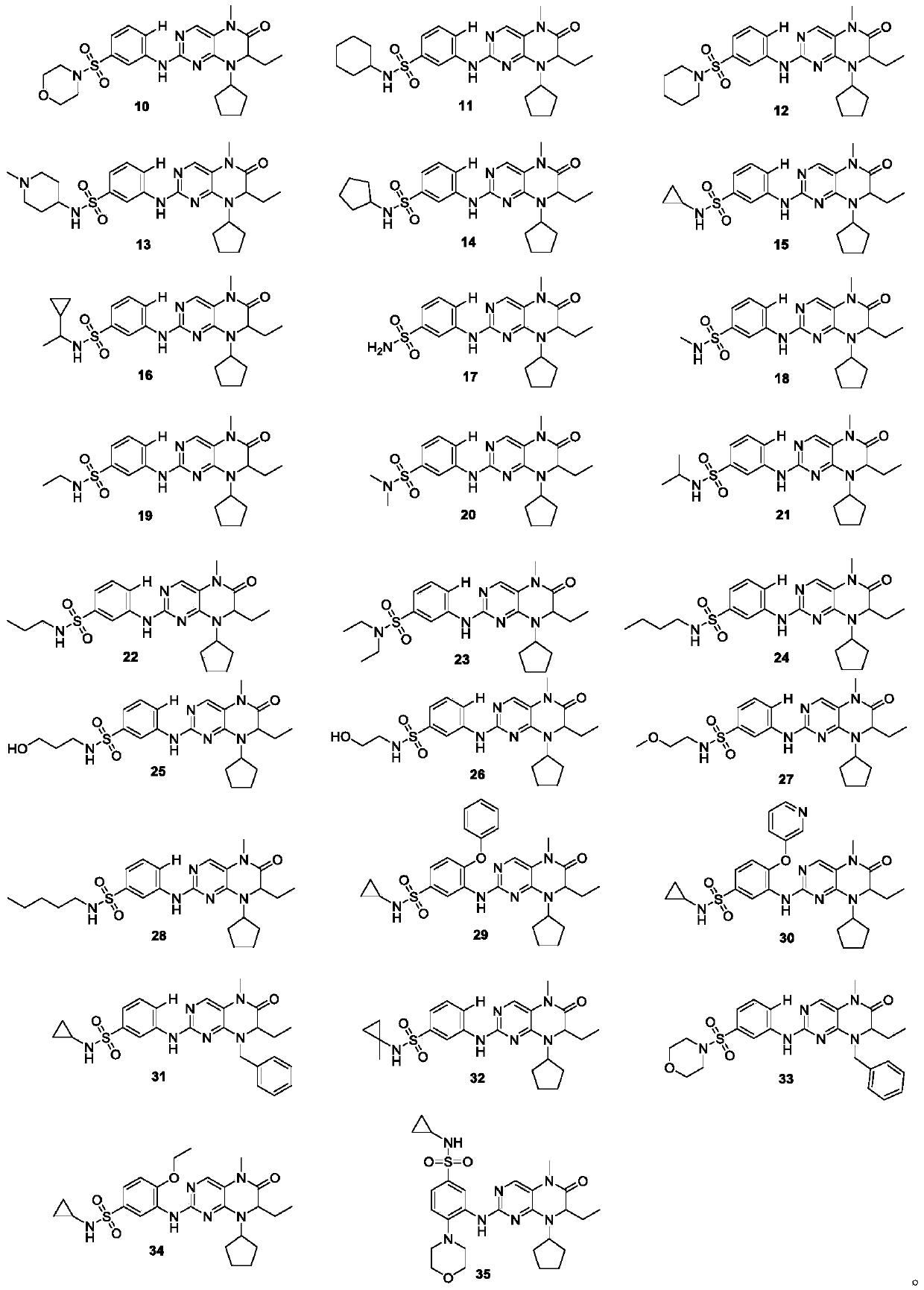
[0091] Example 29: Synthesis of Compound 29
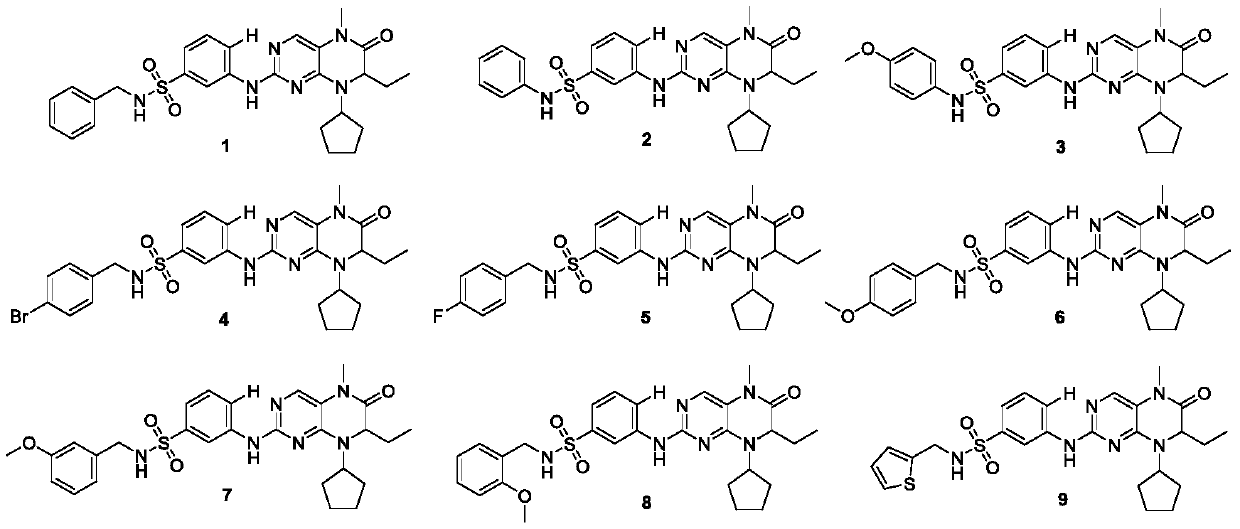
[0092] The preparation methods of compounds 1-35 are the same, and the preparation method of compound 29 is taken as an example.
[0093] With absolute ethanol and 1,4-dioxane as solvent, 40a and 46a prepared above were dissolved in the solvent according to the molar ratio of 1:1, and 2.5 equivalents (relative to 40a) of concentrated hydrochloric acid were added, and heated to reflux. 48h, after the reaction was completed, the solvent was spin-dried, washed with saturated sodium bicarbonate solution, extracted with ethyl acetate, the ethyl acetate phases were combined, concentrated, and the final product 29 was obtained by column chromatography. Yield 76%, pale yellow solid, melting point: 146-147°C. 1 H NMR (400MHz, DMSO-d 6 )δ9.52(s,1H),8.44(s,1H),7.87(s,1H),7.83(d,J=8.0Hz,1H),7.77(d,J=1.8Hz,1H),7.44– 7.31(m, 5H), 7.27(d, J=7.2Hz, 2H), 5.48(d, J=15.3Hz, 1H), 4.36(d, J=15.3Hz, 1H), 4.09(t, J=4.8 Hz, 1H), 3.28 (s, 3H), 2.07 (s,...
Embodiment 1
[0094] Example 1: Synthesis of Compound 1
[0095] The method is the same as in Example 29. Yield 63%, pale yellow solid, melting point: 179-180°C. 1 H NMR (400MHz, DMSO-d 6 )δ9.41(s,1H),8.45(s,1H),8.04(t,J=6.3Hz,1H),7.87–7.80(m,2H),7.43(t,J=8.0Hz,1H), 7.32–7.21 (m, 6H), 4.62–4.51 (m, 1H), 4.23–4.17 (m, 1H), 3.99 (d, J=6.3Hz, 2H), 3.25 (s, 3H), 2.03 (d, J=6.2Hz, 1H), 1.93(d, J=6.4Hz, 1H), 1.71(d, J=10.8Hz, 5H), 1.64–1.55(m, 3H), 0.78(t, J=7.5Hz, 3H). 13 C NMR (100MHz, DMSO-d 6 )δ162.83,154.92,151.58,141.88,140.90,138.53,137.81,129.11,128.17,127.50,127.07,121.30,117.82,115.73,115.60,58.84,57.42,46.15,28.94,28.74,27.78,26.46,23.06,22.66,9.05 .HR-MS(ESI):Calcd.C 27 H 32 N 6 O 3 S,[M+H] + m / z:521.2335,found:521.2334.
Embodiment 2
[0096] Example 2: Synthesis of Compound 2
[0097] The method is the same as in Example 29. Yield 63%, pale yellow solid, melting point: 155-156°C. 1 H NMR (400MHz, DMSO-d 6 )δ10.22(s, 1H), 9.40(s, 1H), 8.44(s, 1H), 7.82(s, 1H), 7.78(d, J=9.5Hz, 1H), 7.37(t, J=8.0 Hz, 1H), 7.25–7.18 (m, 3H), 7.10 (d, J=7.6Hz, 2H), 7.00 (t, J=7.3Hz, 1H), 4.60–4.48 (m, 1H), 4.24–4.18 (m, 1H), 3.25 (s, 3H), 2.03 (d, J=5.9Hz, 1H), 1.91 (d, J=9.3Hz, 1H), 1.78–1.65 (m, 5H), 1.65–1.55 ( m,3H),0.78(t,J=7.5Hz,3H). 13 C NMR (100MHz, DMSO-d 6 )δ162.84,154.80,151.57,141.86,139.93,138.42,137.88,129.11,129.02,123.77,121.66,119.80,117.86,115.79,58.83,57.40,28.93,28.71,27.78,26.46,23.05,22.64,9.04.HR-MS (ESI):Calcd.C 26 H 30 N 6 O 3 S,[M+H] + m / z:507.2178,found:507.2179.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com