Method for splitting aleutric acid enantiomer
A technology of laccarlelic acid and enantiomers, which is applied in the resolution of racemate compounds and the field of laccarlenic acid resolution, can solve the problems of in-depth research, achieve good repeatability, fast peak time, short time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1 Establishment of enantiomer resolution method of laccarlenic acid
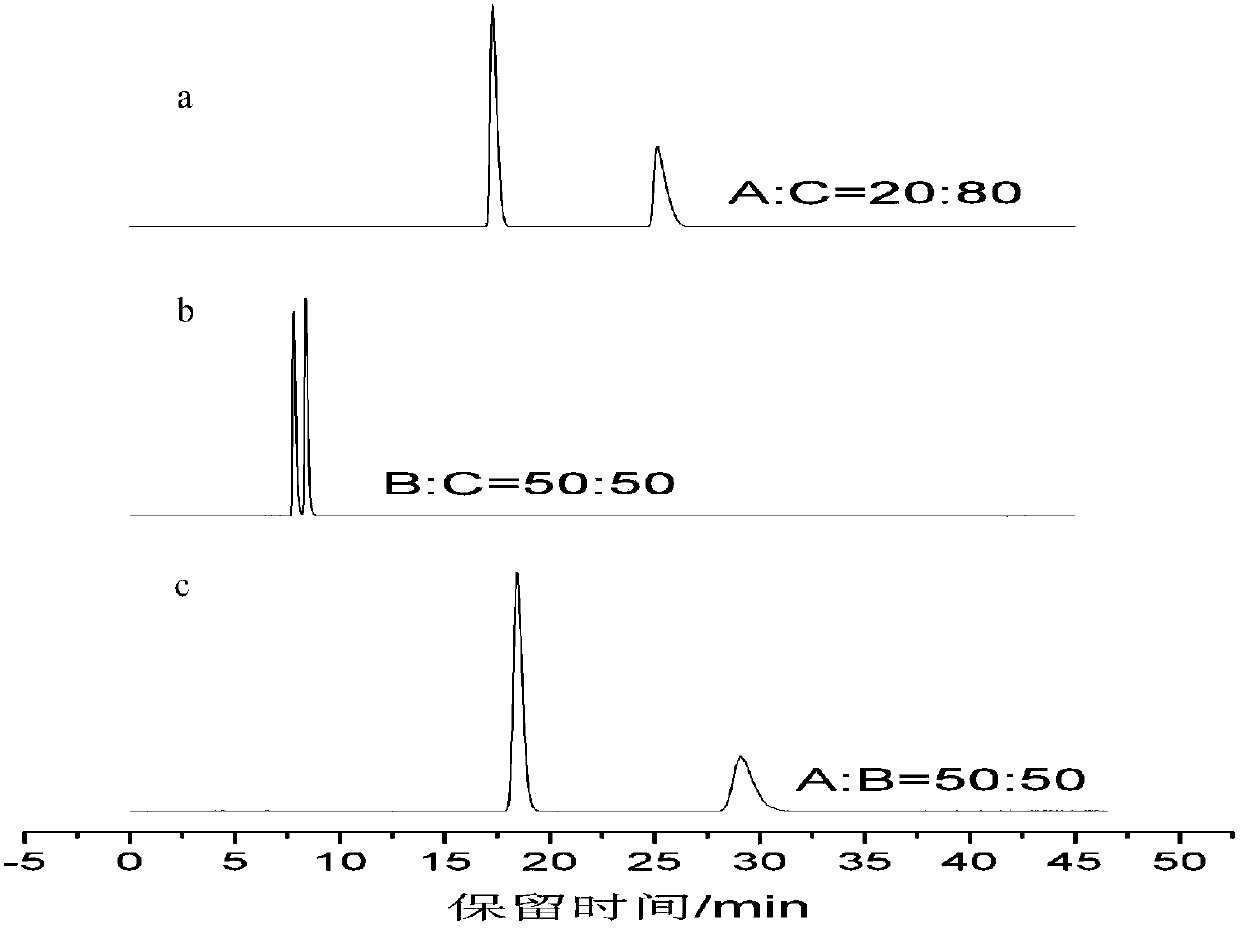
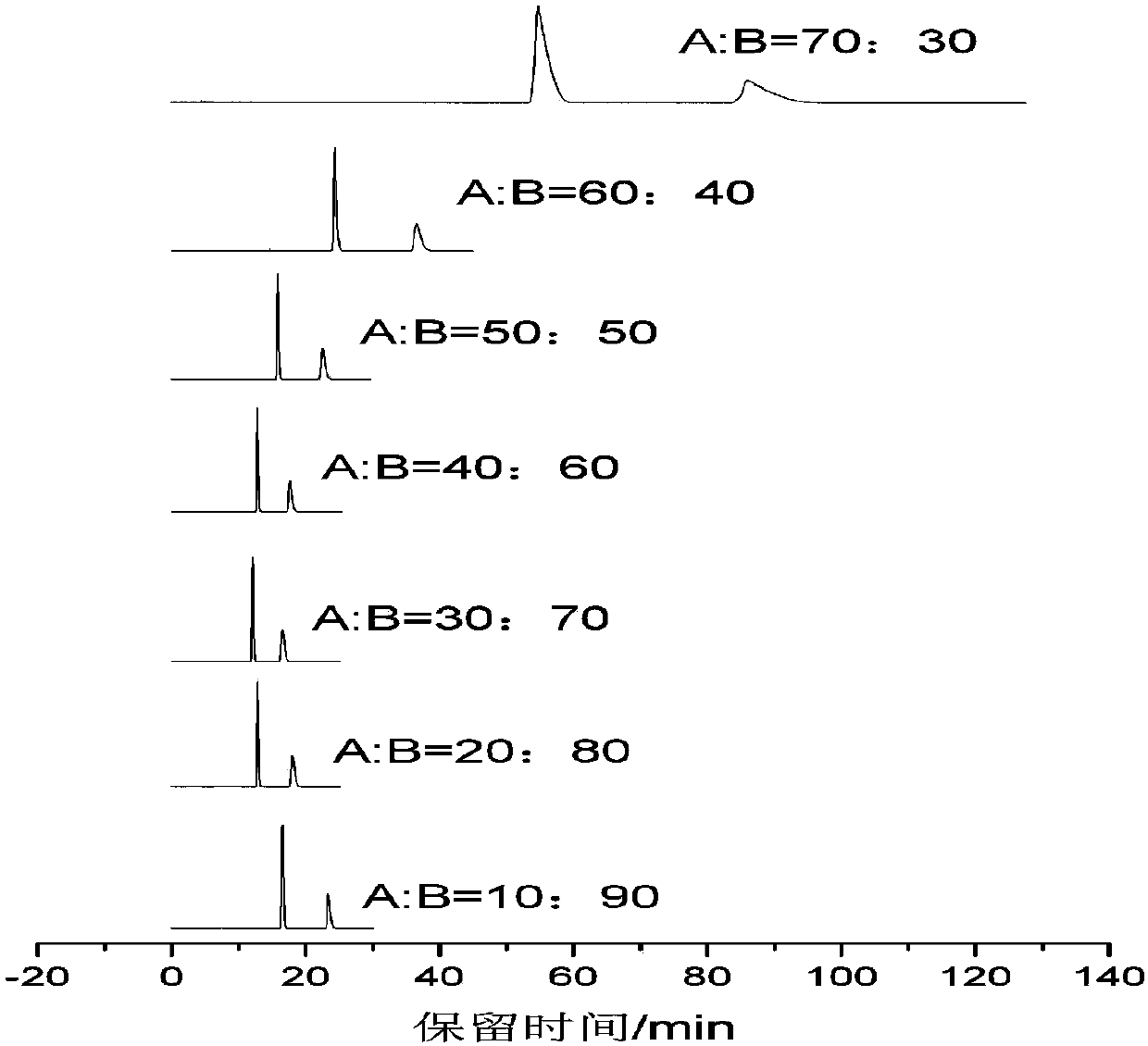
[0055] 1-1) Selection of mobile phase composition
[0056] Accurately weigh 100 mg of a sample of jatrophic acid, dissolve it in methanol, and set the volume to 100 mL to prepare a solution of lac jatrophic acid (i.e., a mixture of lac jatrophic acid) with a concentration of 1 mg / mL, and then dissolve it with 0.45 μm cellulose acetate After filtering through the needle filter membrane, use a micro-sampling needle to draw 5 μL of the sample solution for injection, and inject it into a high-performance liquid chromatograph (Agilent 1200 high-performance liquid chromatography: Agilent Technologies Co., Ltd., USA) for separation, wherein: methanol (C) , acetonitrile (B), 0.1% formic acid aqueous solution (A) are mobile phase components, and mobile phase consists of 0.1% formic acid aqueous solution water: methanol=(20-50):(50-80) (V / V), 0.1% Formic acid aqueous solution: acetonitrile = (20-50)...
Embodiment 2
[0092] Embodiment 2 The content of the enantiomer of laccarpus acid and the determination of ee value
[0093] Prepare 0.5 mg / mL of the lactoic acid helicoid solution, according to the resolution conditions determined in the steps 1-3) of Example 1, split, and adopt the peak area normalization method to measure the prepared lactoic acid enantiomer Enantiomer content, and calculate the enantiomeric excess value (ee value). The calculation method of ee value is as formula (Ⅰ):
[0094]
[0095] In the formula, [a]: the percentage content / % of the first enantiomer; [b]: the percentage content / % of the second enantiomer. The measurement results are shown in Table 5.
[0096] Table 5 Determination table of two enantiomer content and ee value of laccarnation acid
[0097]
[0098] As can be seen from Table 5, under the above-mentioned optimal conditions determined, when the preparation of Lacic acid was carried out to measure the enantiomer content with 0.5mg / mL concentrati...
Embodiment 3
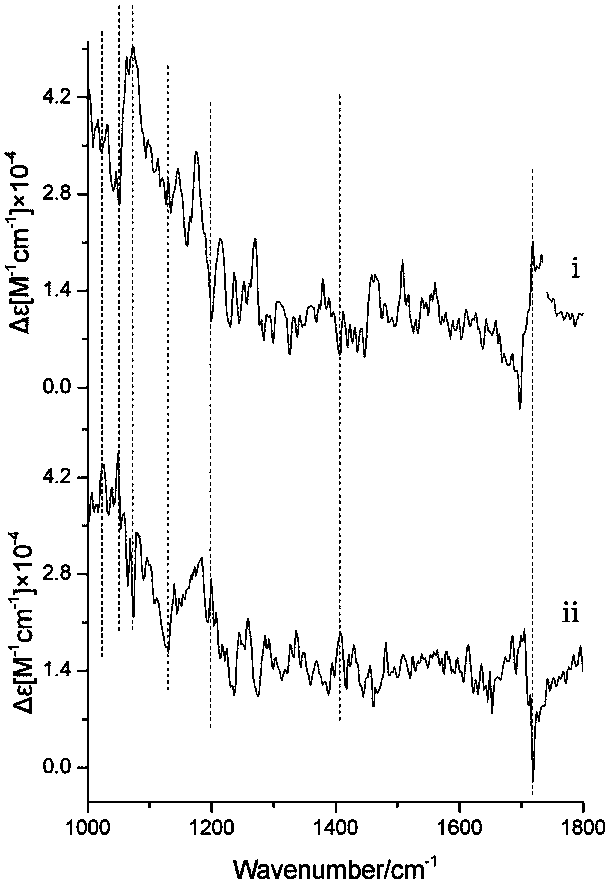
[0099] The infrared spectrum (FTIR) comparative analysis of the laccarlenic acid before and after the resolution of embodiment 3
[0100] Get Lacic acid (Ⅲ) before the resolution and the enantiomer (i, ii) after the resolution, and carry out the infrared absorption spectrum test according to the following conditions respectively: the tabletting substrate is potassium bromide, the pressure is 10MPa, and the infrared scanning wavenumber The range is 4000~400cm -1 , resolution 4cm -1 , accumulatively scanning 32 times.
[0101] Measurement results such as Image 6 shown by Image 6 It can be seen that the infrared absorption characteristics of the heteromorph (Ⅲ) and the enantiomer i and ii samples of lac jaloic acid are basically the same, and it can be seen that the prepared and resolved lac jatrophic acid i and ii samples are consistent with the structure of lac jatrophic acid sex. The main characteristic absorption peaks are assigned as follows: at 3306cm -1 It is the s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com