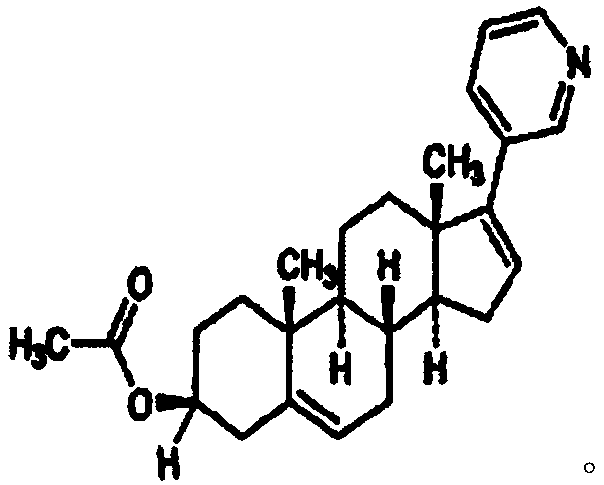
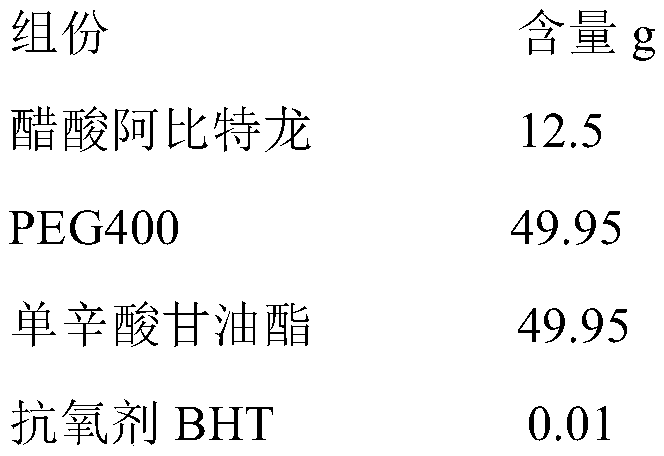
A kind of cyp17 inhibitor soft capsule and preparation method thereof
A technology of capsules and fatty acid glycerides, which is applied in the field of CYP17 inhibitor soft capsules and its preparation, can solve the problems of complex preparation process, lower drug stability, slow drug dissolution, etc., and achieve simple preparation process, increase dissolution rate and bioavailability degree, to avoid the effect of stimulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1) Prescription
[0028] Component content g
[0029] Abiraterone Acetate 10
[0030] Glyceryl monooleate 40
[0031] 2) Preparation process:
[0032] Prescription quantity takes abiraterone acetate and glycerol monooleate, stirs to dissolve and become a uniform clear solution, and fills the solution into soft capsules.
Embodiment 2
[0034] 1) Prescription
[0035] Component content g
[0036] Abiraterone Acetate 10
[0037] Polyoxyethylene Glyceryl Oleate 100
[0038] 2) Preparation process:
[0039] Prescription amount weighs abiraterone acetate and polyoxyethylene olein glyceride, stirs to dissolve into a uniform clear solution, and fills the solution into soft capsules.
Embodiment 3
[0041] 1) Prescription
[0042] Component content g
[0043] Abiraterone Acetate 10
[0044] Macrogol Glyceride Oleate 150
[0045] 2) Preparation process:
[0046] Prescription amount weighs abiraterone acetate and macrogol glyceride oleate, stirs to dissolve and become a uniform clear solution, and fills the solution into soft capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com