A kind of synthetic method of 3-substituted isocoumarin catalyzed by ruthenium
A technology of isocoumarin and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of complex process, high cost, and many synthesis steps, and achieve the effect of simple operation and few reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053]
[0054]The reactor was placed under nitrogen, and 0.40mmol (78.4mg) α-carbonylsulfide ylide (1a), 0.02mmol (12.2mg) [Ru(p-cymene)Cl 2 ] 2 , 0.08mmol (27.5mg) AgSbF 6 , 0.40mmol (24.0mg) AcOH, 2mL TFE, stirred at 100°C for 12h. After the reaction was completed, the solvent was adjusted with a rotary evaporator, and the crude product was subjected to column chromatography with a mixed solvent of petroleum ether and ethyl acetate as the eluent to obtain 40.5 mg of 3-substituted isocoumarin 2a with an isolated yield of 91%.
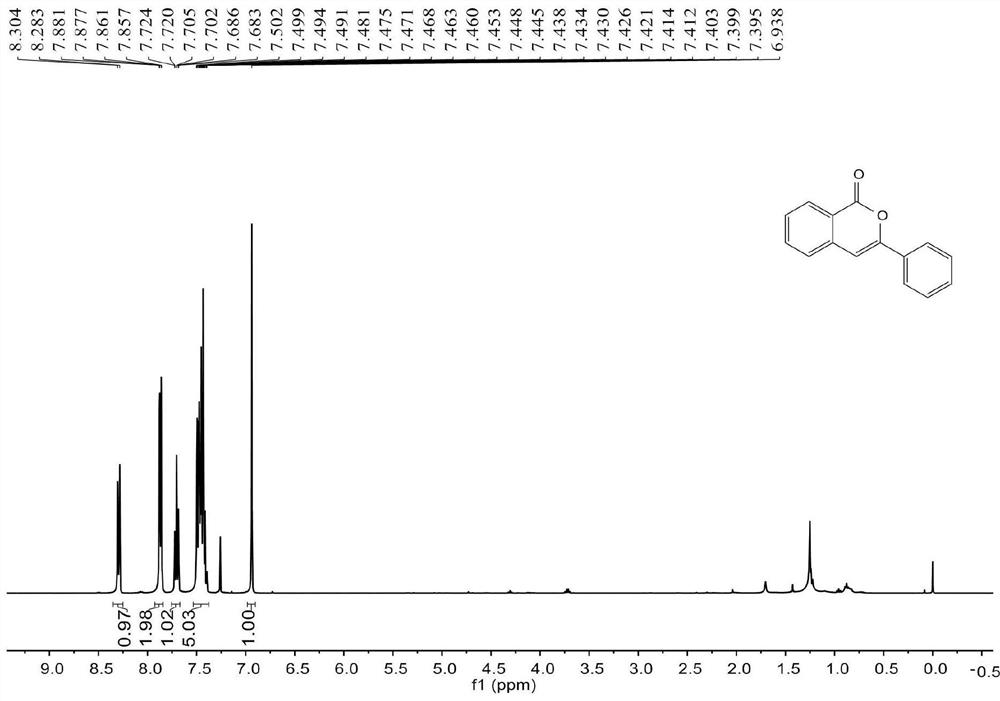
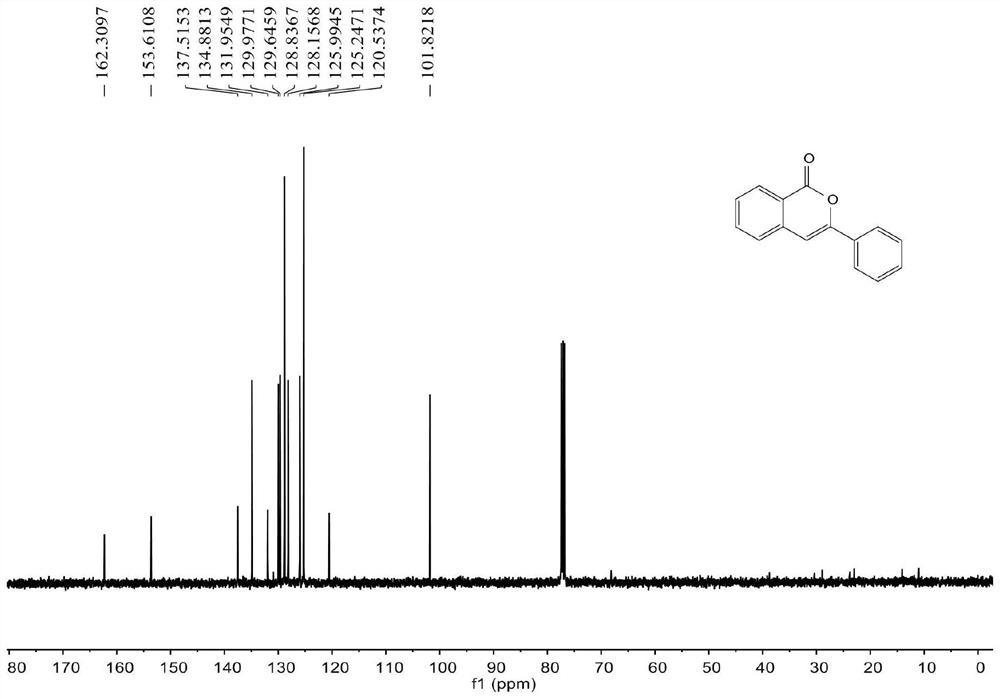
[0055] join figure 1 , figure 2 , the characterization data of compound 2a are as follows:
[0056] 1 H NMR (400MHz, CDCl 3 )δ8.29(d, J=8.4Hz, 1H), 7.87(dd, J=8.0, 1.6Hz, 2H), 7.72-7.68(m, 1H), 7.50-7.40(m, 5H), 6.94(s ,1H). 13 C NMR (100MHz, CDCl 3 ) δ 162.3, 153.6, 137.5, 134.9, 132.0, 130.0, 129.6, 128.8, 128.2, 126.0, 125.2, 120.5, 101.8.
Embodiment 2
[0058]
[0059] The reactor was placed under nitrogen, and 0.40mmol (84.0mg) α-carbonylsulfide ylide (1b), 0.02mmol (12.2mg) [Ru(p-cymene)Cl 2 ] 2 , 0.08mmol (27.5mg) AgSbF 6 , 0.40mmol (24.0mg) AcOH, 2mL TFE, stirred at 100°C for 12h. After the reaction was completed, the solvent was adjusted with a rotary evaporator, and the crude product was subjected to column chromatography with a mixed solvent of petroleum ether and ethyl acetate as the eluent to obtain 45.0 mg of 3-substituted isocoumarin 2b with an isolation yield of 90%.
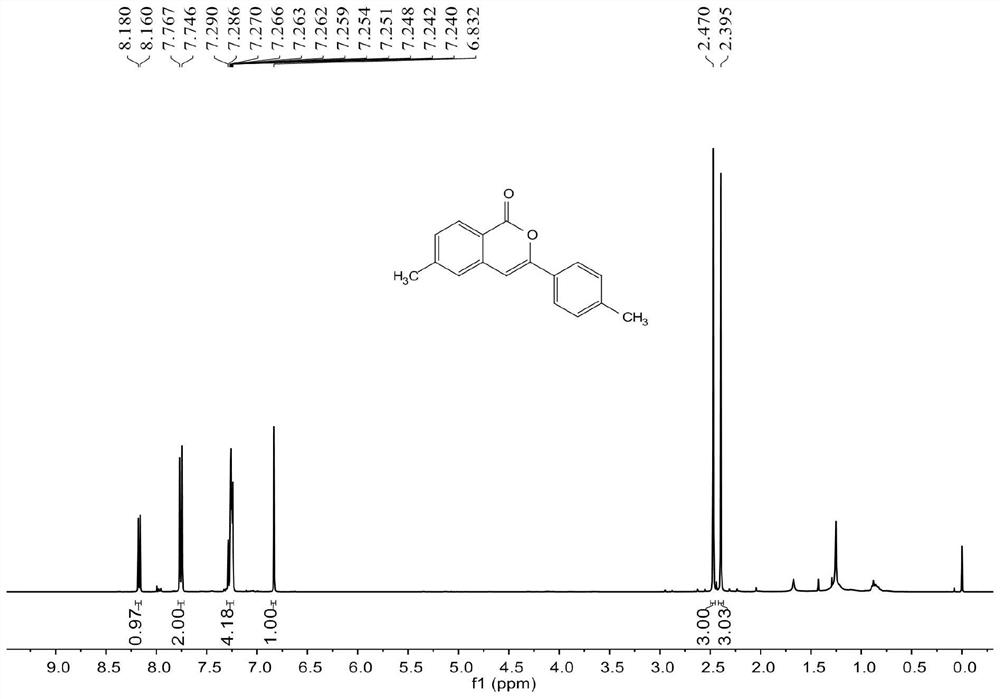
[0060] join image 3 , Figure 4 , the characterization data of compound 2b are as follows:
[0061] 1 H NMR (400MHz, CDCl3) δ8.17 (d, J = 8.0Hz, 1H), 7.76 (d, J = 8.4Hz, 2H), 7.29-7.24 (m, 4H), 6.83 (s, 1H), 2.47 (s,3H),2.40(s,3H). 13 C NMR (100MHz, CDCl3) δ 162.5, 153.9, 145.9, 140.2, 137.8, 129.6, 129.5, 129.4, 129.3, 125.8, 125.1, 118.0, 101.1, 22.0, 21.4.
Embodiment 3
[0063]
[0064] The reactor was placed under nitrogen, and 0.40mmol (92.0mg) α-carbonylsulfide ylide (1c), 0.02mmol (12.2mg) [Ru(p-cymene)Cl 2 ] 2 , 0.08mmol (27.5mg) AgSbF 6 , 0.40mmol (24.0mg) AcOH, 2mL TFE, stirred at 100°C for 12h. After the reaction was completed, the solvent was adjusted with a rotary evaporator, and the crude product was subjected to column chromatography with a mixed solvent of petroleum ether and ethyl acetate as the eluent to obtain 48.9 mg of 3-substituted isocoumarin 2c with an isolation yield of 84%.
[0065] see Figure 5 , Image 6 , the characterization data of compound 2c are as follows:
[0066] 1 H NMR (400MHz, CDCl3) δ8.23(d, J=8.4Hz, 1H), 7.81(d, J=8.4Hz, 2H), 7.49-7.44(m, 4H), 6.86(s, 1H). 13 C NMR (100MHz, CDCl3) δ 161.2, 153.8, 141.7, 138.7, 136.5, 131.4, 130.0, 129.2, 128.9, 126.6, 125.5, 118.8, 101.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com