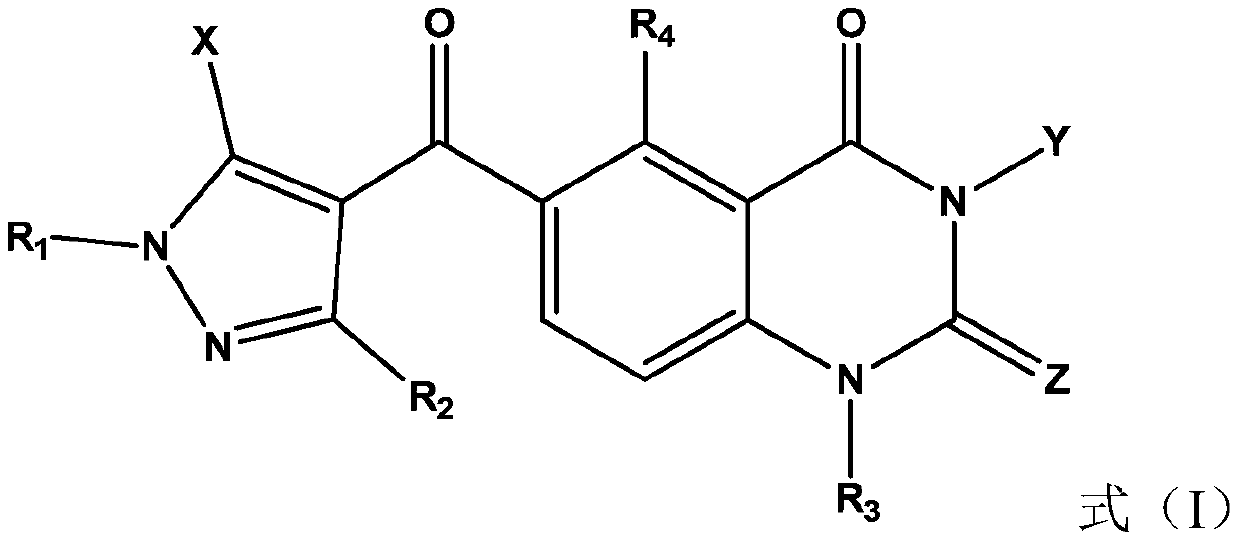
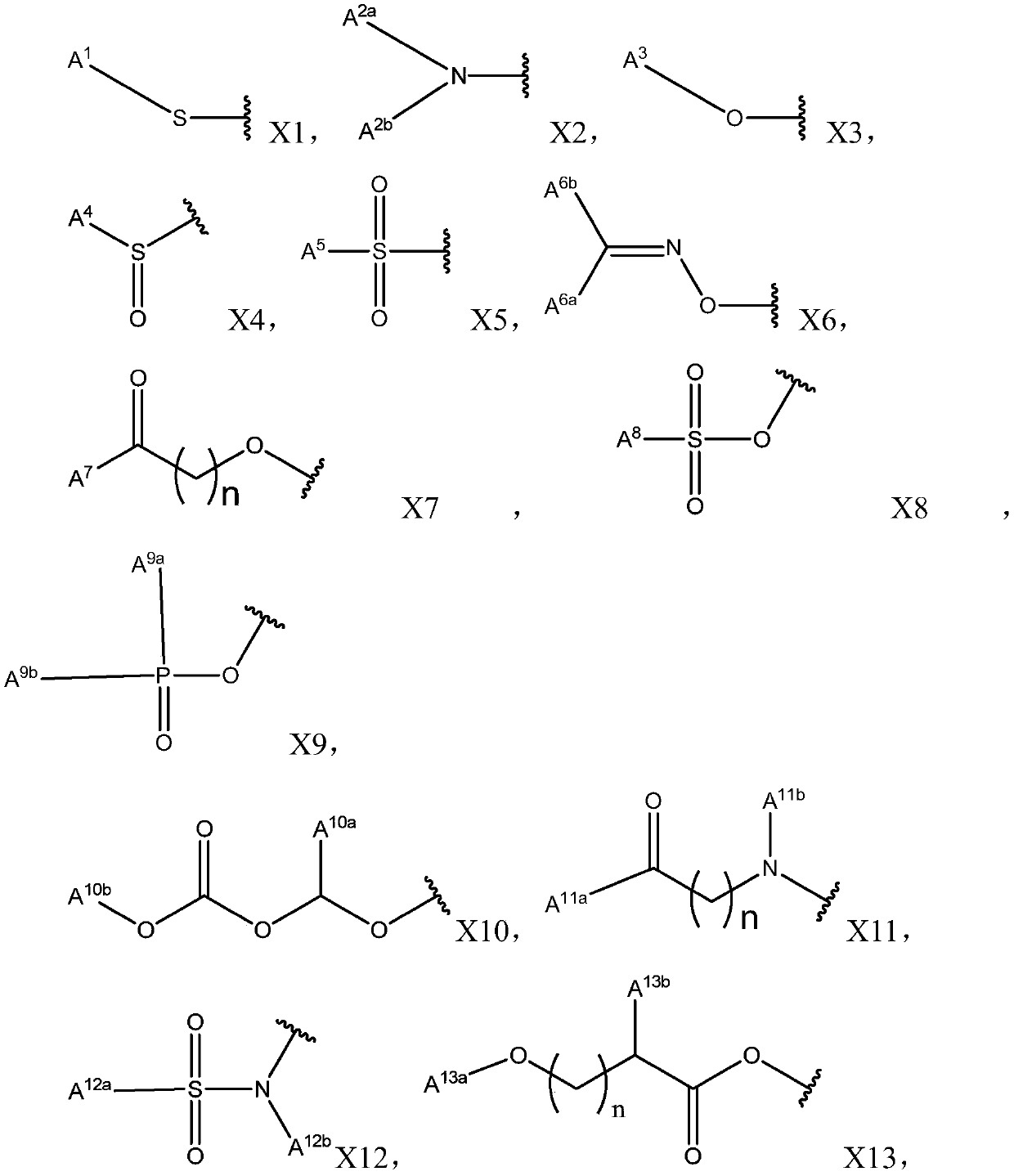
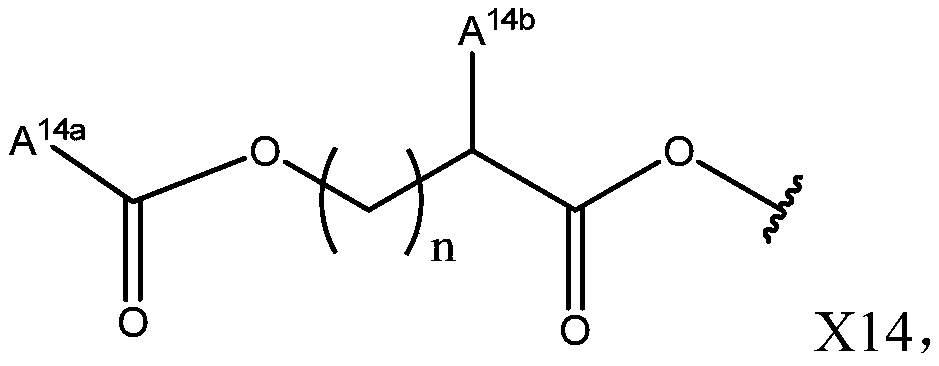
Pyrazole-based derivative containing quinazolinedione fragment, and applications thereof, and pesticide herbicide
A technology of quinazolinediones and pyrazoles, which is applied in the direction of sugar derivatives, sugar derivatives, biocides, etc., can solve the problems of loss of control effect and weed resistance of malignant weeds, and achieve good crops Safety, excellent prevention effect, good safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0055] Preparation Example 1: Synthesis of Compound 1-3a
[0056]
[0057] Add intermediate I-1a (5mmol) to a 50mL single-necked bottle, add 18mL dry THF, and slowly add SOCl dropwise at room temperature 2 (8mmol), after the dropwise addition, reflux at 75°C for about 1.5h, follow the reaction progress by TLC, and remove the solvent after the reaction. Add 20 mL of dry CHCl 3 , 1,3-Dimethyl-5-pyrazolone (6mmol), Et 3 N (10mmol), reacted for about 0.5h, followed by TLC until the acid chloride disappeared. After the reaction is completed, wash once with 20 mL of water, wash twice with 10 mL of 1 mol / L HCl each time, and wash twice with 10 mL of saturated NaHCO each time. 3 Wash 2 times, anhydrous Na 2 SO 4 Dry and pass through the column to obtain intermediate I-2b.
[0058] Intermediate I-2b (1mmol) was added to a 50mL two-necked bottle, 28mL of anhydrous acetonitrile was added, and N 2 Join Et under protection 3 N (2 mmol), acetone cyanohydrin (0.1 mmol). The react...
preparation example 2
[0059] Preparation Example 2: Synthesis of Compound 8
[0060] At 0°C, dissolve compound I-3a (1.5mmol) in anhydrous dichloromethane, add triethylamine (3mmol), stir and dissolve, then add cyclopropylformyl chloride (2mmol) to the reaction system, continue Stir for 3-6h. After the reaction was completed, a saturated sodium bicarbonate solution (15 ml) was slowly added to the reaction system, the reaction solution was extracted with dichloromethane, and the organic phase was washed twice with saturated brine. The organic phase was dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain a crude product, which was then separated and purified by column chromatography.
preparation example 3
[0061] Preparation Example 3: Synthesis of Compound 46
[0062] At 0 °C, intermediate I-3a (1 mmol) was dissolved in ultra-dry DCM, and Et 3 N (1.5mmol) was stirred for 15min, and a solution of 2-thiophenesulfonyl chloride (1.5mmol) was slowly added dropwise to the reaction system, stirring was continued at 0°C for 30min, and then returned to room temperature for 3h. After the reaction was completed, a saturated sodium bicarbonate solution (15 ml) was added to the reaction system to separate and collect the organic phase, which was washed twice with saturated brine. The organic phase was dried with anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain a crude product, which was then separated and purified by column chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com