Anion metal organic framework material for sensing nitro aromatic hydrocarbon explosive as well as preparation method and application of anion metal organic framework material
A technology of metal-organic framework and anion framework, applied in the direction of organic chemical methods, 2/12 group organic compounds without C-metal bonds, analytical materials, etc., to achieve the expansion of types and application fields, simple synthesis methods, and high product purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
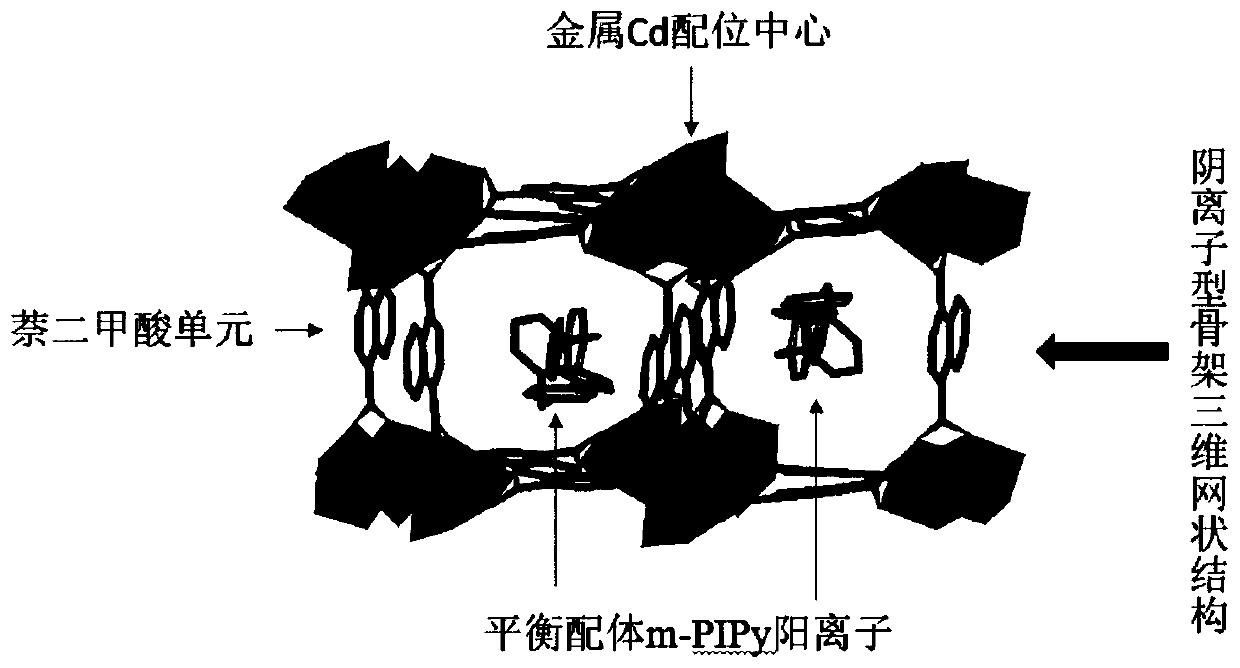
[0035] The embodiment of the present invention also provides a method for preparing an anionic framework metal-organic framework material for sensing p-nitroaromatic explosives, comprising the following steps: 1,3-bis([4-(1-imidazolyl)-pyridine Base]-methylene)benzenedibromide (m-PIPy), 1,4-naphthalene dicarboxylic acid (NDC) and cadmium nitrate were reacted in a solvent to synthesize a metal-organic framework material with an anionic framework.
[0036] In the process of preparing the metal-organic framework material of the above-mentioned anionic framework, the present application uses 1,3-bis([4-(1-imidazolyl)-pyridyl]-methylene)benzenedibromide (m-PIPy) As a ligand, a metal-organic framework material with an anionic framework was synthesized.
[0037] In the embodiments of the present invention, the solvent is an inorganic solvent and / or an organic solvent well known to those skilled in the art, and the present application is not particularly limited. For example, the solv...
Embodiment 1
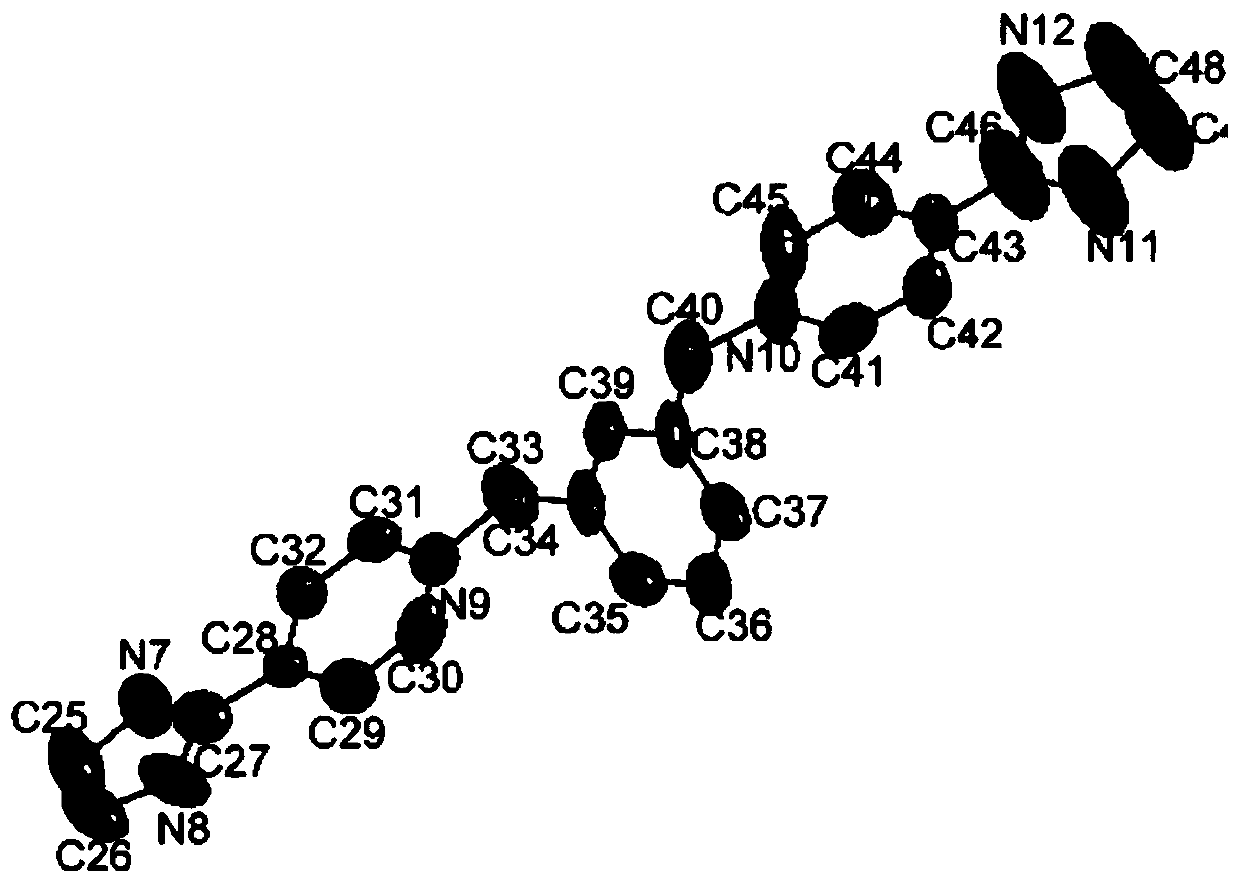
[0045] The synthesis of ligand m-PIPy, the synthetic route is as follows:
[0046]
[0047] Add 4-PIM (10mmol, 1.45g), m-dibromobenzyl (5mmol, 1.32g), methanol (10mL) into a 50mL round bottom flask, and stir at 78°C for 18h. Stop the reaction and cool to room temperature. Spin to dry methanol, add K to the reaction solution 2 CO 3 Solution (K 2 CO 3 :H 2 O=1.3g:20g), a large amount of solids were produced, suction filtered, recrystallized from ethanol, and dried to obtain 3.26g of yellow solid powder with a yield of 59%.
[0048] Yield: 59%, m.p.: 155-180°C; ESI-MS: [M–2Br - ] 2+ m / z=197.35, (Calcd: 197.23)
[0049] IR(ν max , KBr, cm -1 ):3405m, 1632vs, 1529m, 1479m, 1435m, 1392s, 1280w, 1171m, 1061m, 1032m, 953w, 859w, 755w, 712w.
[0050] 1 H NMR (300MHz, DMSO-d 6 ), δ=8.74(d, J=6.8Hz, 4H), 8.24(d, J=6.8Hz, 4H), 7.52(m, 4H), 7.50(s, 4H), 5.64(s, 4H)
[0051] 13 C NMR (75MHz, DMSO-d 6 ), δ (ppm) = 150.7, 149.3, 148.8, 142.5, 136.9, 136.6, 130.4, 128.8, 128...
Embodiment 2
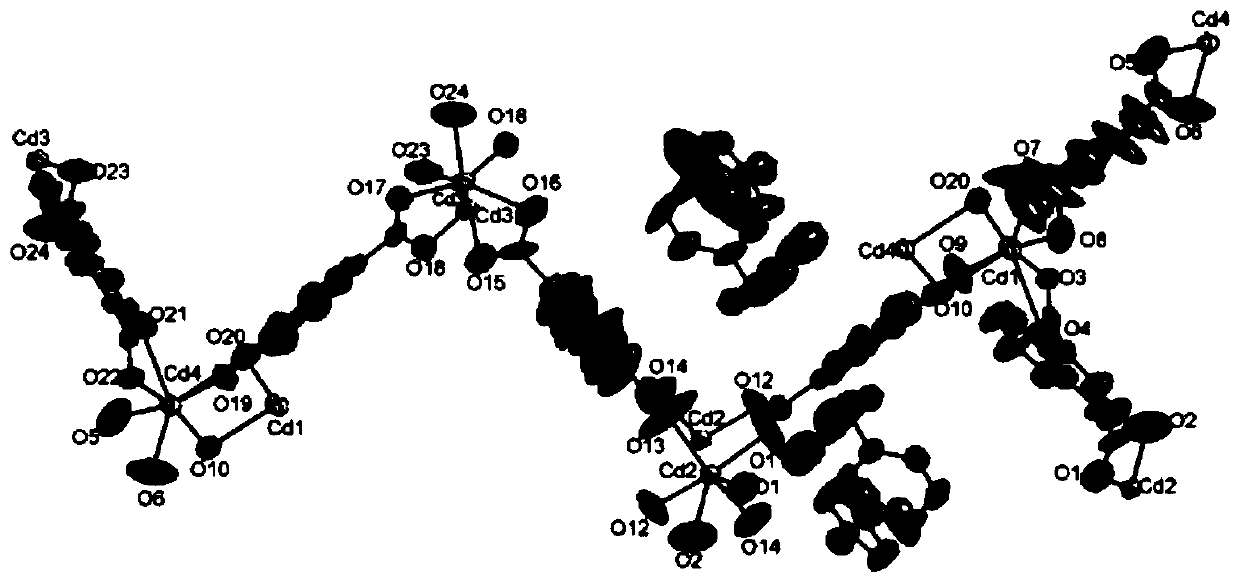
[0053] Cd 2 (m-PIPy)(NDC) 3 Synthesis of: Add Cd(NO 3 ) 2 4H 2 O (0.2mmol, 45.7mg), 1,4-naphthalene dicarboxylic acid (0.2mmol, 43.5mg), m-PIPy (0.1mmol, 39.2mg), NaOH (0.4mmol, 16mg), methanol (3mL), H 2 After O (5mL), seal it and place it in the reactor, use an oven to raise the temperature of the mixture in the reactor to 170°C at a rate of 2°C / min, keep it warm for 3 days (4320min), and then heat it at a rate of 3°C / h Cool down to room temperature at a rate of 100°C to obtain yellow transparent block crystals; filter the yellow transparent block crystals, wash with ethanol and water, and dry in vacuo to obtain Cd 2 (m-PIPy)(NDC) 3 , the yield was 51%. IR(ν max , KBr, cm -1 ):3432m, 2924m, 1635s, 1563s, 1510s, 1479s, 1406s, 1358s, 1261m, 1165m, 1114m, 1030w, 938w, 832m, 788m, 711m.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com