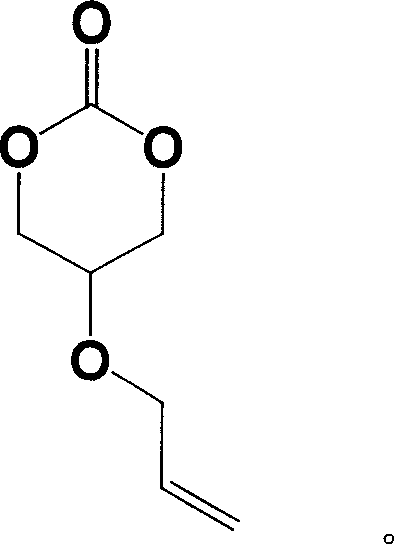
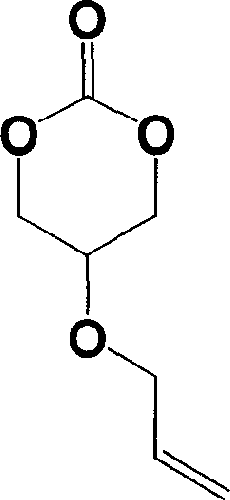
5-acrylic oxo-trimethylene carbonate and its preparation and use
A technology of methyl carbonate and allyloxy, applied in the direction of organic chemistry, can solve the problems of high hydrophobicity, slow degradation performance, tissue cytocompatibility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
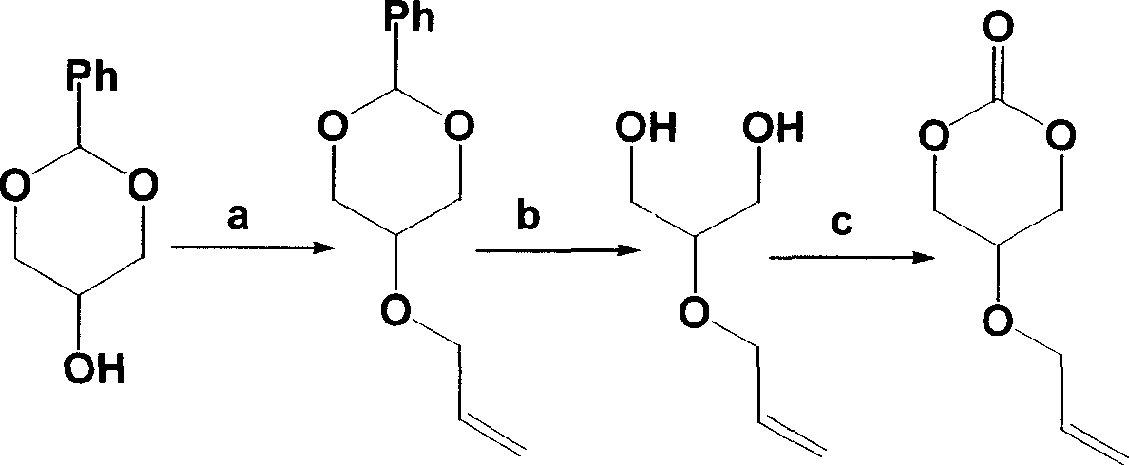
Embodiment 1
[0027] Preparation of 5-allyloxy-1,3-benzylidene glycerol: 18.0 g of 5-hydroxy-1,3-benzylidene glycerol and 100 ml of anhydrous tetrahydrofuran were placed in a 250 ml round bottom flask, and 6.0 g of hydrogenated Sodium, stirred for 8 hours, added 18 ml of allyl bromide, stirred for 24 hours. After filtration, the solvent was evaporated and dried to obtain 20.3 g of 5-allyloxy-1,3-benzylidene glycerol as a white solid, with a yield of 92%. The melting point is 50°C. Infrared spectrum 1645cm -1 (-CH 2 CHCH 2 ).
Embodiment 2
[0029] Preparation of 2-allyloxy-1,3-propanediol: 23.0 g of 5-allyloxy-1,3-benzylidene glycerol, 50 ml of methanol and 50 ml of 1M aqueous hydrochloric acid were placed in a 250 ml round bottom flask , reflux reaction for 24 hours. Pour into saturated potassium carbonate solution, extract with ethyl acetate, and distill off the solvent to obtain 12.8 g of colorless liquid 2-allyloxy-1,3-propanediol with a yield of 93%. Proton NMR spectrum ( 1 H NMR) (CDCl 3 , ppm): 5.90 (m, 1H, -CH 2 CH=CH 2 ), 5.30 (m, 2H, -CH 2 CH=CH 2 , 4.10 (d, 2H, O=COCH 2 CH-), 3.60(d, 2H, -OCH 2 CHCH 2 ), 3.46 (s, 1H, O=COCH 2 CH).
Embodiment 3
[0031] Preparation of 5-allyloxy-trimethylene carbonate: 13 g of 2-allyloxy-1,3-propanediol, 38 g of antipyrine and 200 ml of anhydrous THF were placed in a 500 ml round bottom flask In 10 ml of a solution of 7 g of triphosgene in tetrahydrofuran was added, and the reaction was carried out at 20° C. for 24 hours. After filtration, the solvent was distilled off to obtain 7.8 g of colorless liquid 5-allyloxy-trimethylene carbonate, with a yield of 50%. Proton NMR spectrum ( 1 H NMR) (CDCl 3 , ppm): 5.90 (m, 1H, -CH 2 CH=CH 2 ), 5.30 (m, 2H, -CH 2 CH=CH 2 ), 4.48 (s, 2H, O=COCH 2 CH-), 4.12(d, 2H, -OCH 2 CHCH 2 ), 3.90 (s, 1H, O-COCH 2 CH). Carbon-13 NMR spectrum ( 13 C NMR) (CDCl 3 , ppm): 147 (C=O), 133 (-OCH 2 CH=CH 2 ), 17 (-OCH 2 CH=CH 2 ), 77 (O=COCH 2 CH-), 70(-OCH 2 CHCH 2 ), 66 (O=COCH 2 CH). Infrared spectrum 1754cm -1 (C=O).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com