Synthesis method of 4,4-difluoropiperidine-1-formyl chloride
A technique for the synthesis of difluoropiperidine and its synthesis method, which is applied in the field of synthesis of 4,4-difluoropiperidine-1-formyl chloride, and can solve the problems of unstable raw materials and products, hazards to humans and the environment, and low reaction yields , to achieve the effects of simple post-treatment, high total yield and simple reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A kind of synthetic method of 4,4-difluoropiperidine-1-formyl chloride, prepare through the following steps:
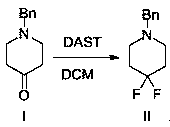
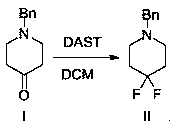
[0026] Step 1: Add benzylpiperidone (100.0g, 528.4mmol) and dichloromethane (1.0 L) into a three-neck flask at room temperature, then add DAST (85.2g, 528.4mmol) dropwise at 0±5°C ). After the addition, the temperature was raised to room temperature and reacted at room temperature for 10-20 hours; after the reaction was completed, quenched with water (500 mL), added concentrated hydrochloric acid (50 mL), separated the aqueous phase, and washed the organic phase with water (500 mL); combined the aqueous phases, Basified with sodium carbonate, then extracted with dichloromethane (500mL×2), the obtained organic phase was dried over anhydrous magnesium sulfate, concentrated and purified to obtain the target product compound II 1-benzyl-4,4-difluoropiperidine 97.1g (87.0% yield, 98% purity).
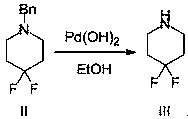
[0027] The second step: at room temperature, the 1-benzyl-4,4-difluoro...
Embodiment 2
[0033] A kind of synthetic method of 4,4-difluoropiperidine-1-formyl chloride, prepare through the following steps:
[0034] Step 1: Add benzylpiperidone (100.0g, 528.4mmol) and dichloromethane (1.0 L) into a three-neck flask at room temperature, then add DAST (127.8g, 792.6mmol) dropwise at 0±5°C ), after the addition, the temperature was raised to room temperature and reacted at room temperature for 10-20 hours. After the reaction was completed, quenched with water (500 mL), added concentrated hydrochloric acid (50 mL), separated the aqueous phase, washed the organic phase with water (500 mL), combined with water Phase was basified with sodium carbonate, and then extracted with dichloromethane (500mL×2). The obtained organic phase was dried over anhydrous magnesium sulfate, concentrated and purified to obtain 98.2g of the target product (yield 88.0%, purity 98%).
[0035] The second step: at room temperature, the 1-benzyl-4,4-difluoropiperidine (98.0g, 463.9mmol) prepared in...
Embodiment 3
[0039] A kind of synthetic method of 4,4-difluoropiperidine-1-formyl chloride, prepare through the following steps:
[0040] Step 1: Add benzylpiperidone (100.0g, 528.4mmol) and N,N-dimethylformamide (1.0 L) into a three-necked flask at room temperature, then add DAST dropwise at 0±5°C (255.5g, 1585.2mmol), after the addition, the temperature was raised to room temperature and reacted at room temperature for 10-20 hours; after the reaction was completed, quenched with water (500mL), added concentrated hydrochloric acid (50mL), separated the aqueous phase, and washed the organic phase with water (500mL), the combined aqueous phase was alkalized with sodium carbonate, then extracted with dichloromethane (500mL×2), the obtained organic phase was dried over anhydrous magnesium sulfate, concentrated and purified to obtain 96.0g of the target product (yield 86.0%, purity 98%).
[0041]The second step: at room temperature, the 1-benzyl-4,4-difluoropiperidine (98.0g, 463.9mmol) obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com