Diarylethene compound as well as medicine composition and application thereof
A technology of diarylethene and compound, which is applied in the field of medicine and achieves the effects of small side effects, chemical ease and novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Part of the diarylethene compounds of the present invention are purchased from Holland specs company (website: http: / / www.specs.com), and the corresponding numbers of the diarylene compounds in the library are as shown in Table 1:
[0058] Table 1 is the corresponding number of some diarylethene compounds in the library
[0059] compound Numbering compound Numbering compound Numbering 66 AO-289 / 25117029 75 AB-131 / 06806028 93 AJ-291 / 34003011 67 AM-814 / 41094729 76 AA-516 / 12432405 94 AG-777 / 36178018 68 AB-131 / 42301429 78 AG-690 / 37079203 103 AA-504 / 06813049 69 AB-131 / 42300921 80 AN-970 / 40920809 107 AT-051 / 43422454 70 AB-131 / 42300746 82 AB-131 / 42302837 108 AE-641 / 02580034 71 AB-131 / 40897212 84 AI-211 / 09901015 114 AG-664 / 02670043 72 AB-131 / 40897213 85 AG-690 / 11665343 116 AE-641 / 01920053 73 AB-131 / 40897214 88 AG-690 / 12135048 117 AE-641 / 02517058 74 AB-131 / 4089...
Embodiment 2
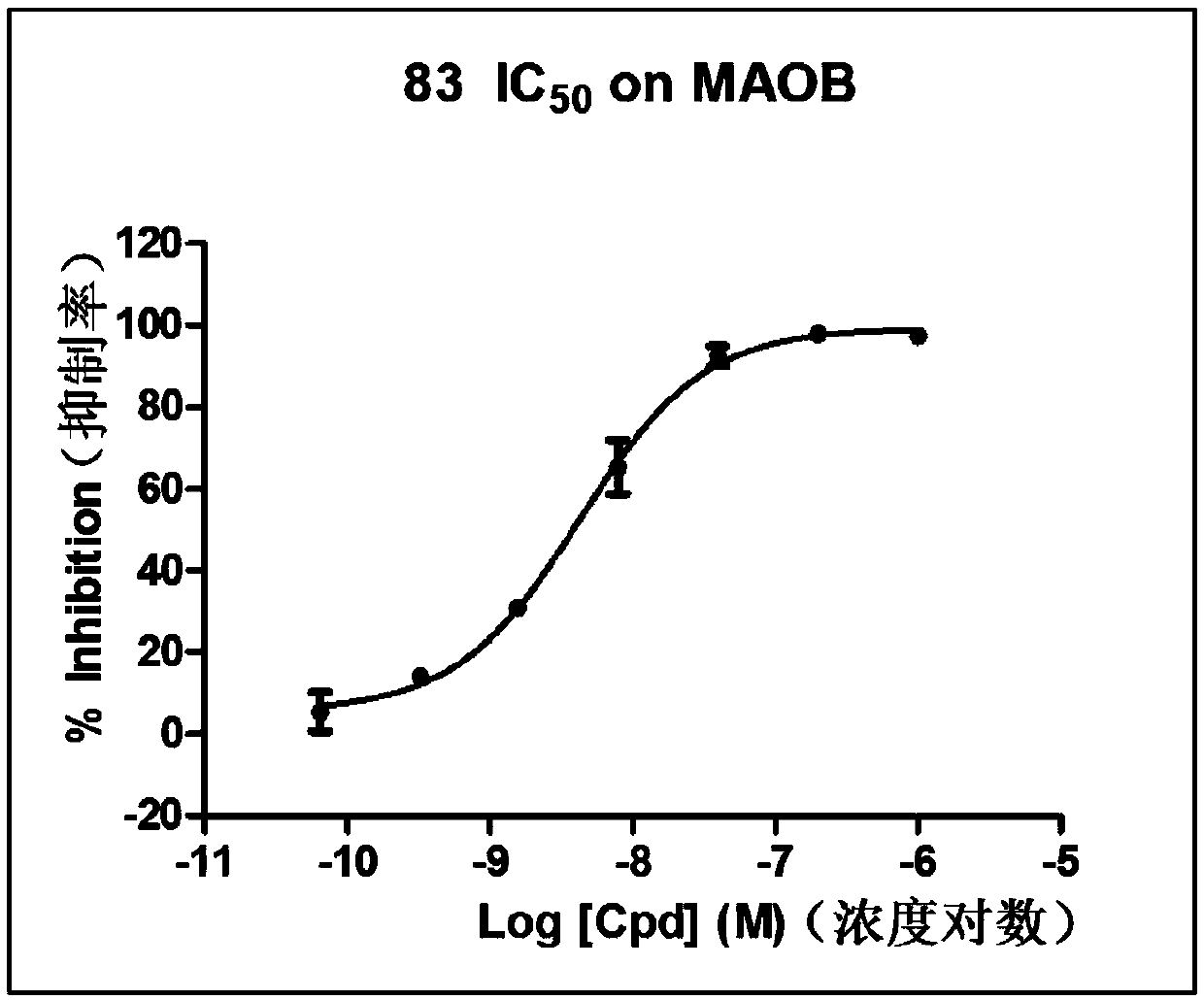
[0061] This example mainly provides the preparation method of compound 83 and the structure determination data of compound 83.
[0062] The English name of compound 83 is as follows:
[0063] (Z)-2-(4-chlorophenyl)-3-[4-(trifluoromethyl)phenyl]prop-2-enenitrile
[0064] The preparation method of compound 83 is as follows:
[0065]
[0066] Specific preparation steps: add 455mg p-chlorophenylacetonitrile, 0.41mL p-trifluoromethylbenzaldehyde and 21mg sodium ethoxide to 10mL methanol, stir at room temperature for 2 hours, filter to obtain a white solid, wash twice with 2mL low-temperature ethanol, and dry to obtain 349mg Compound 83 (38% yield).
[0067] The structure determination data of compound 83 are as follows:
[0068] 1 H NMR (400MHz, CDCl 3 )δ7.97(d, J=8.0Hz, 2H), 7.74(d, J=8.0Hz, 2H), 7.63(d, J=8.8Hz, 2H), 7.55(s, 1H), 7.45(d, J=8.8Hz, 2H).
Embodiment 3
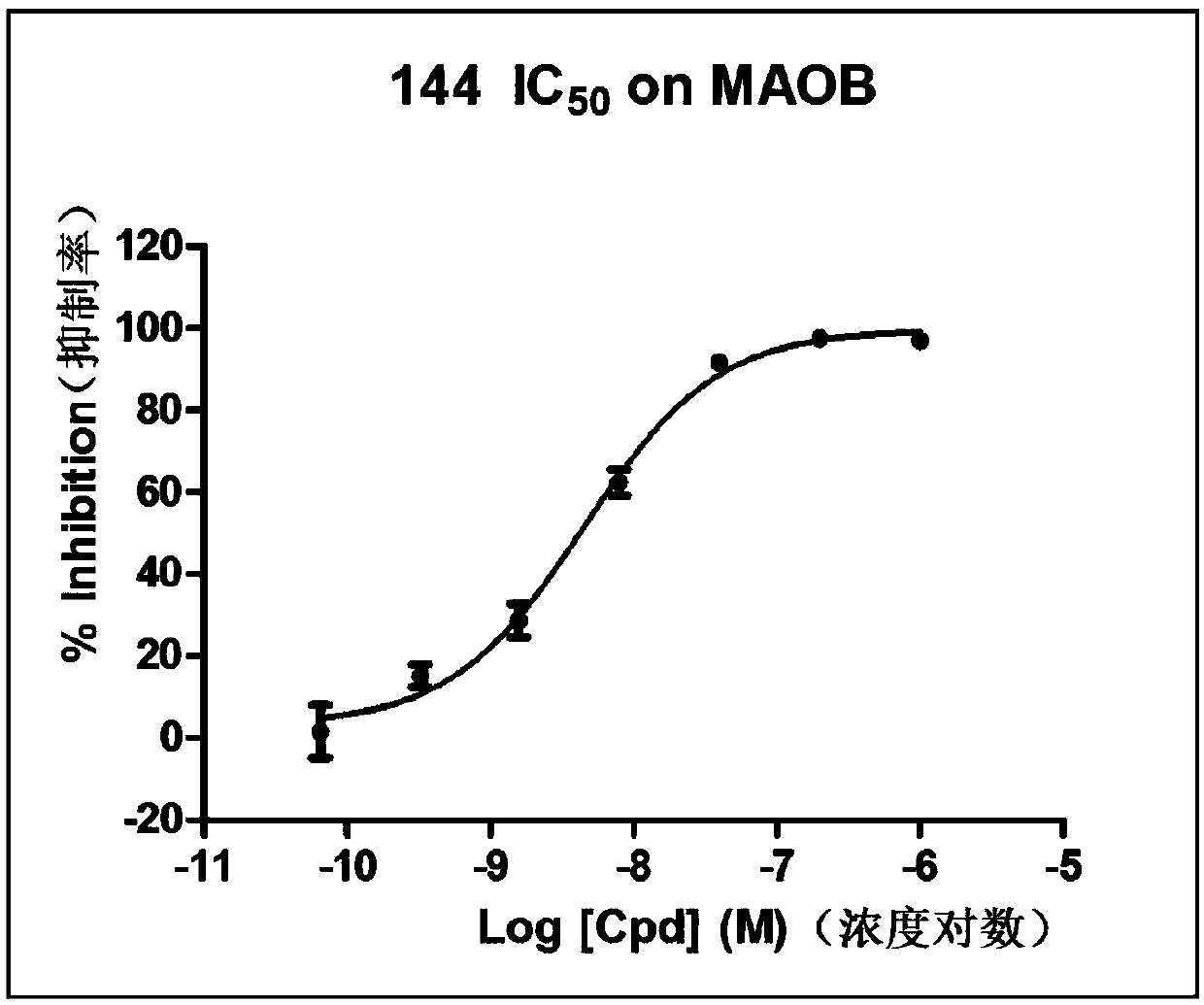
[0070] This example mainly provides the preparation method of compound 144 and the structure determination data of compound 144.
[0071] The English name of compound 144 is as follows:
[0072] (Z)-2-(4-chlorophenyl)-3-[6-(trifluoromethyl)-3-pyridyl]prop-2-enenitrile
[0073] The preparation method of compound 144 is as follows:
[0074]
[0075] Specific preparation steps: just replace the reaction raw material in the preparation step of compound 83: p-trifluoromethylbenzaldehyde with 6-(trifluoromethyl)pyridine-3-carbaldehyde.
[0076] The structure determination data of compound 144 are as follows:
[0077] 1 H NMR (400MHz, CDCl 3 )δ8.94(s,1H),8.62(d,J=8.4Hz,1H),7.82(d,J=8.4Hz,1H),7.66(d,J=8.8Hz,2H),7.56(s, 1H), 7.48 (d, J=8.8Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com