Method for fully synthesizing natural product cerbinal and application ofcerbinal
A natural product, total synthesis technology, applied in the field of pesticides, can solve problems such as mass preparation of unfavorable natural products, research on unfavorable biological activity, easy deterioration, etc., and achieve the effects of easy industrial amplifying synthesis, shortening the synthesis route, and improving the total yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
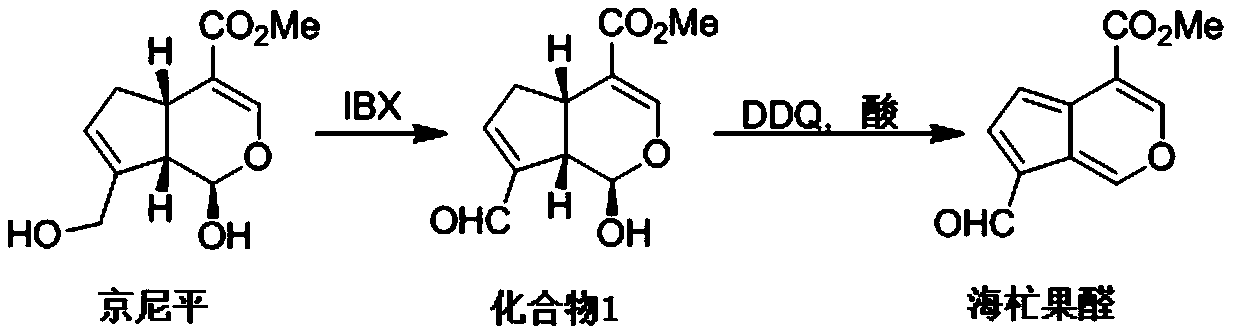
[0020] Total synthetic route of mangolin:
[0021]
[0022] Concrete preparation steps are as follows:
[0023] 8.00 g (35.4 mmol) of genipin, 10.89 g (38.9 mmol) of 2-iodobenzoic acid (IBX) and 200 ml of dimethyl sulfoxide were added to a 500 mL round bottom flask. Under argon protection, after electromagnetic stirring at room temperature for 3 h, 300 mL of water was added. Suction filtration, the filtrate was extracted four times with ethyl acetate, the organic phases were combined, washed with water, washed with saturated brine, dried over anhydrous magnesium sulfate, suction filtered, and precipitated to obtain the crude product of 1, and the pure product of compound 1 was obtained by column chromatography. It is light yellow solid.
[0024] In 250mL round bottom flask, add compound 1 (1.00g, 4.46mmol), DDQ (1.01g, 4.46mmol), CF 3 SO 3 H (0.67g, 4.46mmol), 100mL dichloromethane. Stir at room temperature, TLC tracking reaction complete, after cooling, add sodium car...
Embodiment 2
[0026] The second step of the total synthetic route of mangolin can also be carried out by the following method:
[0027]
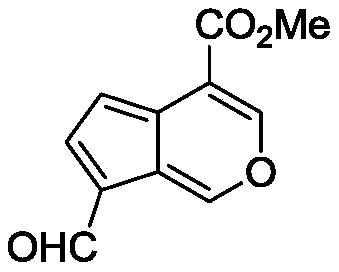
[0028] Compound 1 (1.00 g, 4.46 mmol), DDQ (1.06 g, 4.68 mmol), TsOH (0.77 g, 4.46 mmol), and 100 mL of toluene were added to a 250 mL round bottom flask. Heat and stir at 100°C, after TLC traces the complete reaction, add aqueous sodium carbonate solution after cooling, separate liquids, wash the aqueous phase twice with ethyl acetate, combine the organic phases, wash once with water, wash once with saturated sodium chloride, anhydrous magnesium sulfate Drying, suction filtration, precipitation, and column chromatography yielded the natural product marmandal, which was a bright yellow solid with a yield of 76%.
Embodiment 3
[0030] The second step of the total synthetic route of mangolin can also be carried out by the following method:
[0031]
[0032] Compound 1 (1.00g, 4.46mmol), DDQ (1.32g, 5.80mmol), trifluoroacetic acid (5.09g, 44.6mmol), and 100mL 1,2-dichloroethane were added to a 250mL round bottom flask. Heat and stir at 80°C, after TLC traces the complete reaction, add aqueous sodium carbonate solution after cooling, separate the liquids, wash the aqueous phase twice with ethyl acetate, combine the organic phases, wash once with water, wash once with saturated sodium chloride, anhydrous magnesium sulfate Drying, suction filtration, precipitation, and column chromatography yielded the natural product marmandal, which was a bright yellow solid with a yield of 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com