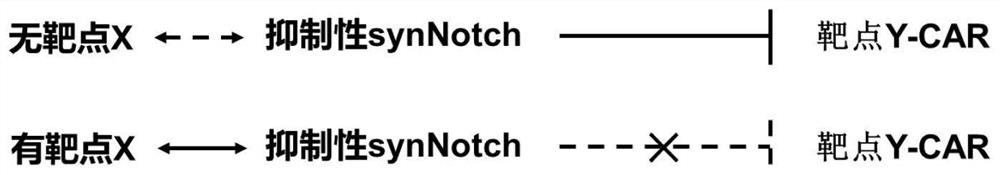
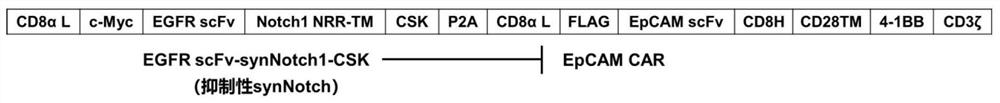
A kind of inhibitory synthetic notch and dual target system and its preparation method and application
A dual-target, inhibitory technology, applied in the field of genetic engineering, can solve problems such as off-target toxicity of CAR-T cells, and achieve the effects of solving off-target toxicity, improving accuracy, and responding quickly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] According to the amino acid sequence of the dual-target system, the corresponding gene sequence was synthesized, the codons were humanized and optimized, and a BamHI restriction site was added at the 5'-end, and a SalI restriction site was added at the 3'-end. Take 4 μg DNA containing the gene of the dual-target system, add 2 μL 10×NEBuffer 3.1, 14 μL ddH 2 O, mix well, then add 1 μL BamHI and 1 μL SalI, mix well, and place in a 37°C incubator for 4 hours. Using the same method, take 2μg pWPT-GFP plasmid, add 2μL 10×NEBuffer 3.1, 14μL ddH 2 O, mix well, then add 1 μL BamHI and 1 μL SalI, mix well, and place in a 37°C incubator for 4 hours. The double-enzyme-digested DNA fragments were prestained with Sybergreen, transferred to 1% agarose gel sample wells, and electrophoresed at 80V. Under UV light, the target band is cut, recovered and purified. Take 1 μg each of the double-enzyme-digested DNA fragment of the dual-target system gene and the pWPT-GFP plasmid, add 1 μL...
Embodiment 2
[0075] The culture medium of 293T cells and HUH-7 cells is DMEM medium containing 10% fetal bovine serum and 100×diluted penicillin and streptomycin. The culture medium of HepG2 cells and SK-HEP-1 cells is MEM medium containing 10% fetal bovine serum and 100×diluted penicillin and streptomycin. Jurkat T cell medium is RPMI-1640 medium containing 10% fetal bovine serum and 100 μg / mL gentamicin sulfate. The above cells were placed at 37°C, 5% CO 2 cultured in an incubator. 293T, HepG2, HUH-7 and SK-HEP-1 cells were digested and passaged with 0.25% trypsin.
[0076] Take 5 μg each of the dual-target system pWPT-GFP expression plasmid, psPAX2 and pMD2.G plasmids, and dissolve them in 480 μL 1×HBS (HEPES buffered saline solution). Take 36 μL PEI (polyethyleneimine, 2.5 mg / mL, pH7.4), dilute it to 360 μL with 1×HBS, add it dropwise to the plasmid solution, mix well while adding, and let stand for 20 minutes. The plasmid-PEI mixture was added dropwise to the 10 cm culture dish in...
Embodiment 3
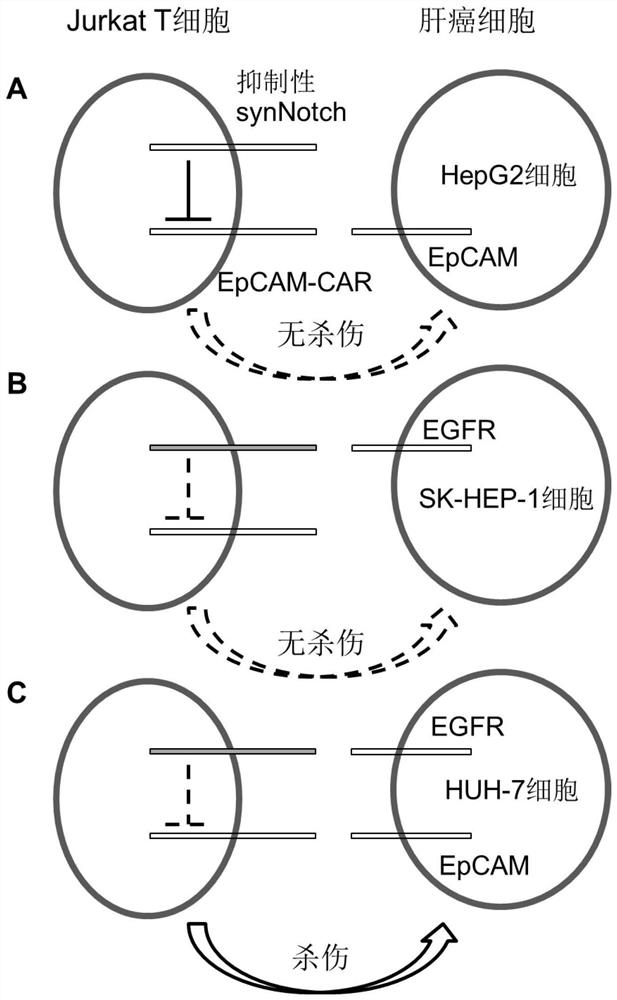
[0080]293T, SK-HEP-1, HepG2, and HUH-7 cells were sucked out of the medium, and 1 mL of 0.25% trypsin was added for digestion. After the cells were digested completely, the medium containing fetal bovine serum was added to stop the digestion, centrifuged at 400×g for 3 minutes, washed twice with PBS, and 500 μL PBS was added to suspend the cells. Add 5 μL of FITC-mouse anti-EGFR IgG1 antibody, FITC-mouse anti-EpCAM IgG1 antibody or FITC-mouse IgG1 isotype control antibody, and incubate at 4°C for 0.5 hours in the dark. Wash twice with PBS, suspend cells with 500 μL PBS, and perform flow cytometry detection. Test results such as Figure 5 As shown, 293T cells are EGFR-EpCAM-, SK-HEP-1 cells are EGFR+EpCAM-, HepG2 cells are EGFR-EpCAM+, HUH-7 cells are EGFR+EpCAM+, which meet the requirements of the dual-target system killing experiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com