New purpose of perazine hydrochloride type compound
A perazine hydrochloride compound and the technology of perazine hydrochloride are applied in the new application field of the compound, and can solve the problems of poor specificity, small number of small molecular compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
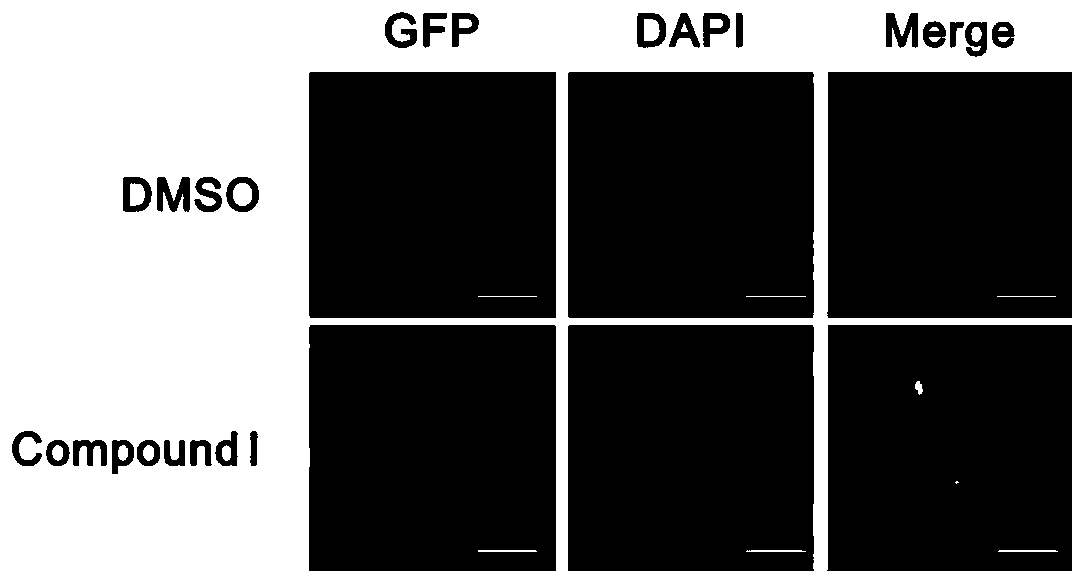
[0029] Screening of Drugs to Promote Nuclear Importation of TFEB
[0030] ① When the TFEB-GFP stable cell line grows to the required density in the 6cm culture dish, the 96-well plate can be plated. Cells were collected, counted on a hemocytometer, and the concentration of the cell suspension was adjusted. 100 μL of the cell suspension was added to each well, plated so that the density of the cells to be tested was 8000 / well, and the edge of the 96-well plate was filled with PBS.
[0031] ②5% CO 2 After incubating at 37°C for 24 h, a final concentration of 10 μM of the drug (i.e. compound I10-[3-(4-methylpiperazin-1-yl)propyl]-2-(trifluoromethyl)phenothiazine disalt was added salt).
[0032] ③ 6 hours after dosing, fix with 4% paraformaldehyde at room temperature for 15-20 minutes.
[0033] ④ Discard 4% paraformaldehyde, wash with PBS three times, take photos with a fluorescent microscope or store at 4°C for subsequent photos.
[0034] like figure 1 The results show that ...
Embodiment 2
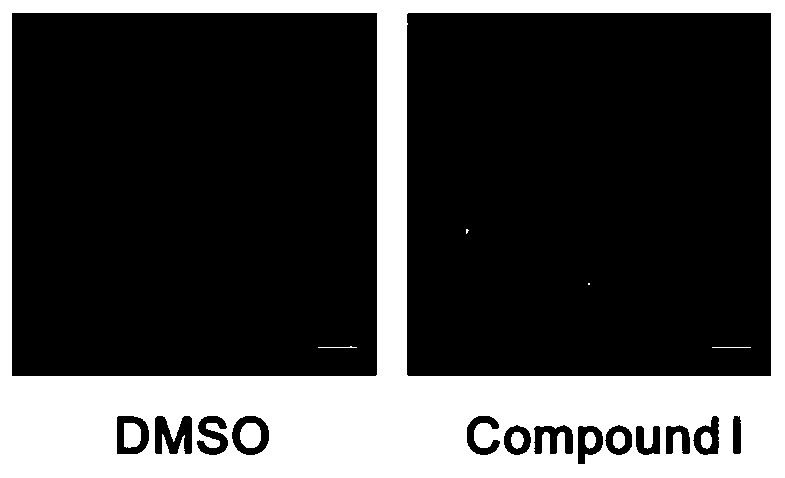
[0036] Screening for autophagy agonists
[0037] ① When the GFP-LC3 stable cell line grows to the desired density in the 6cm culture dish, the 96-well plate can be plated. Cells were collected, counted on a hemocytometer, and the concentration of the cell suspension was adjusted. 100 μL of the cell suspension was added to each well, plated so that the density of the cells to be tested was 8000 / well, and the edge of the 96-well plate was filled with PBS.
[0038] ②5% CO 2 After culturing at 37°C for 24 h, a final concentration of 10 μM drug (i.e. compound I10-[3-(4-methylpiperazin-1-yl)propyl]-2-(trifluoromethyl)phenothiazine disalt was added salt).
[0039] ③ 6 hours after dosing, fix with 4% paraformaldehyde at room temperature for 15-20 minutes.
[0040] ④ Discard 4% paraformaldehyde, wash with PBS three times, take photos with a fluorescent microscope or store at 4°C for subsequent photos.
[0041] like figure 2 The results showed that compound I could significantly i...
Embodiment 3
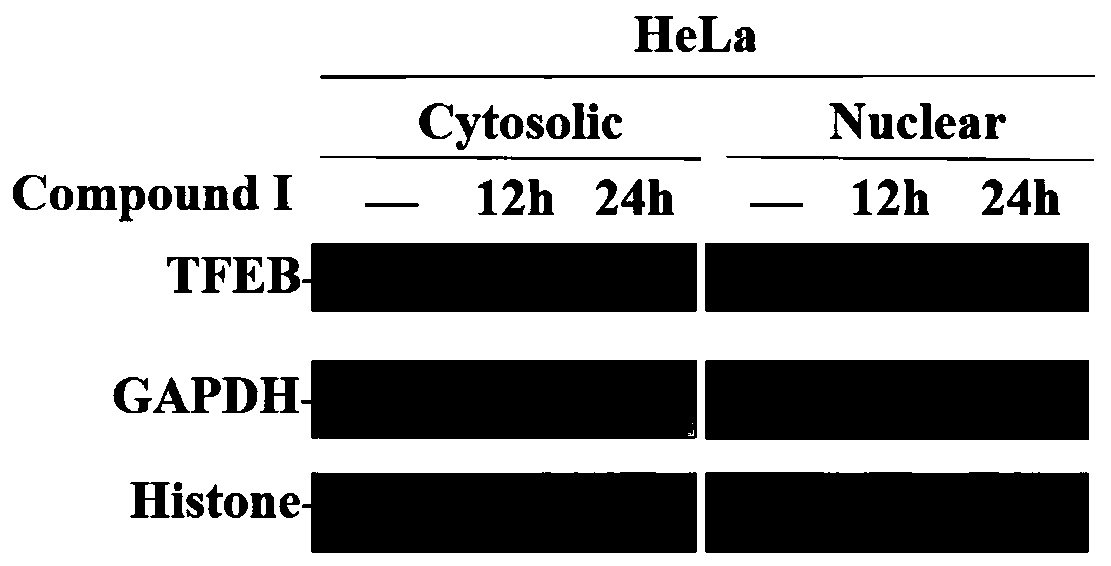
[0043] Effects of Compounds on Nuclear Importation Level of TFEB in HeLa Cells Detected by Nuclear Plasma Separation Technique
[0044] Using the cytoplasmic protein extraction reagents CER I and CER II, under the condition of low osmotic pressure, the cells are fully expanded, and then the cell membrane is destroyed to release the cytoplasmic protein, and then the nucleus is precipitated by centrifugation. The precipitate is the nucleus and the supernatant for cytoplasmic proteins.
[0045]① Inoculate an appropriate amount of cells in a 6-well plate and place at 37°C, 5% CO 2 conditions overnight.
[0046] ②Treat the cells with small molecule compounds and place at 37°C, 5% CO 2 Conditions continue to grow.
[0047] ③For adherent cells, digest the cells with trypsin containing EDTA, then add complete medium to stop the digestion, transfer to a 1.5mL centrifuge tube, centrifuge at 3000rpm for 5min, and collect the cells.
[0048] ④ Wash the collected cells once with PBS, p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com