Method for preparing methyl-2-butenal
A technology of prenaldehyde and prenaldehyde, applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve the loss of raw materials, increase energy consumption of separation process, and reduce prenaldehyde Yield and other issues, to achieve the effect of speeding up the rate, improving the selectivity, and improving the economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
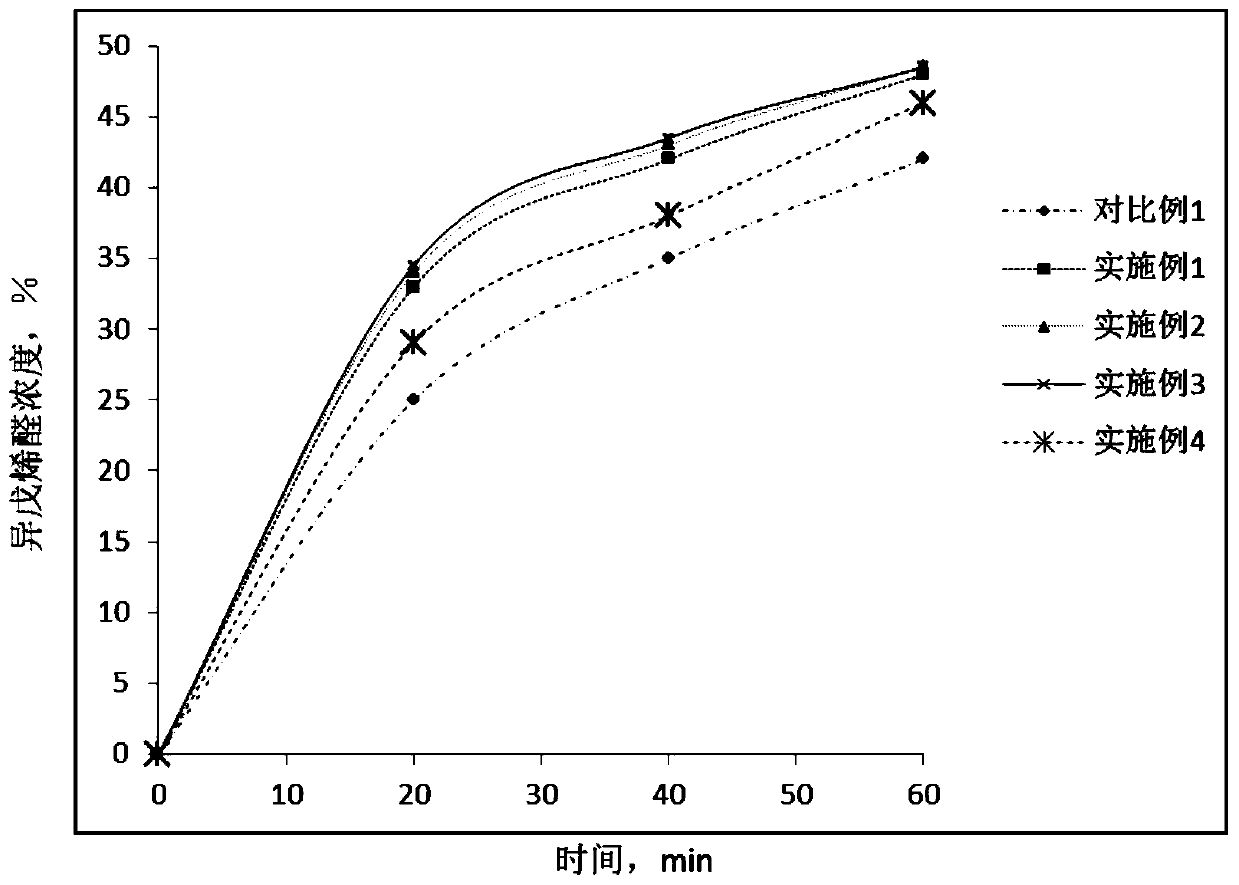
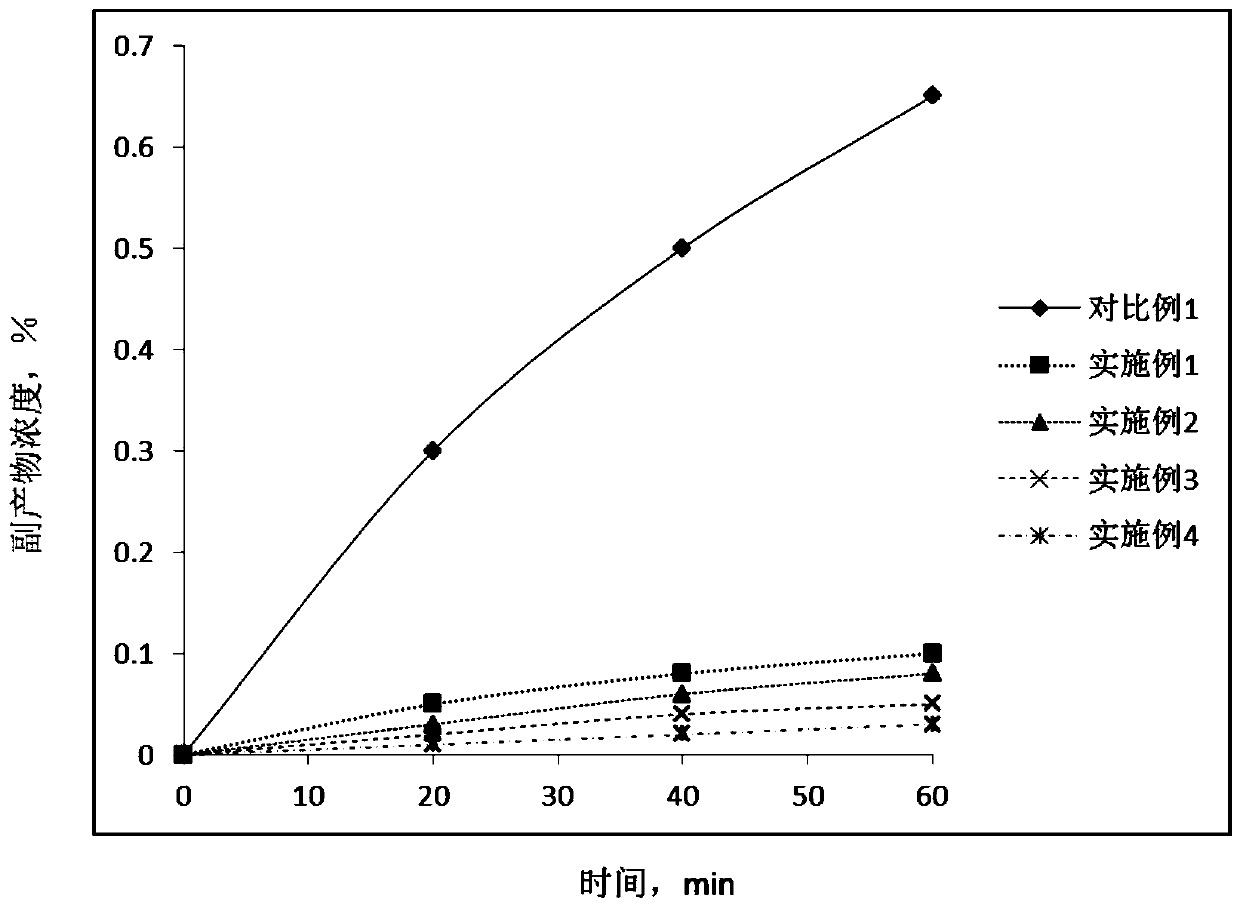
[0053] Add 0.4g of 25wt% ammonia water to the organic phase with a mass ratio of prenol and prenal of 1:1 and a total mass of 1kg, add 1g of prenol to the above mixture, and repeat the experiment of Comparative Example 1 .
[0054] Samples of the reaction mixture were taken at different time points during the reaction, and the concentration of each component of the reaction mixture was measured by chromatography (based on the total mass of the reaction mixture, excluding catalysts and auxiliary agents). The results are shown in Table 1.
Embodiment 2
[0056] Add 0.4g of 25wt% ammonia water to the organic phase with a mass ratio of prenol and prenal of 1:1 and a total mass of 1kg, add 10g of prenol to the above mixture, and repeat the experiment of Comparative Example 1 .
[0057] Samples of the reaction mixture were taken out at different time points during the reaction, and the concentration of each component of the reaction mixture was measured by chromatography (in terms of the total mass of the mixture after the reaction, excluding catalysts and auxiliary agents). The results are shown in Table 1.
Embodiment 3
[0059] Add 0.4g of 25wt% ammonia water to the organic phase with a mass ratio of prenol and prenal of 1:1 and a total mass of 1kg, add 30g of prenol to the above mixture, and repeat the experiment of Comparative Example 1 .
[0060] Samples of the reaction mixture were taken at different time points during the reaction, and the concentration of each component of the reaction mixture was measured by chromatography (based on the total mass of the reaction mixture, excluding catalysts and auxiliary agents). The results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com