Preparing method of Avitinib
A technology of avitinib and tinib, applied in the field of preparation of avitinib, can solve problems such as toxicity and poor effect of T790M mutation, and achieve the effects of simple operation, high yield and optimized reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
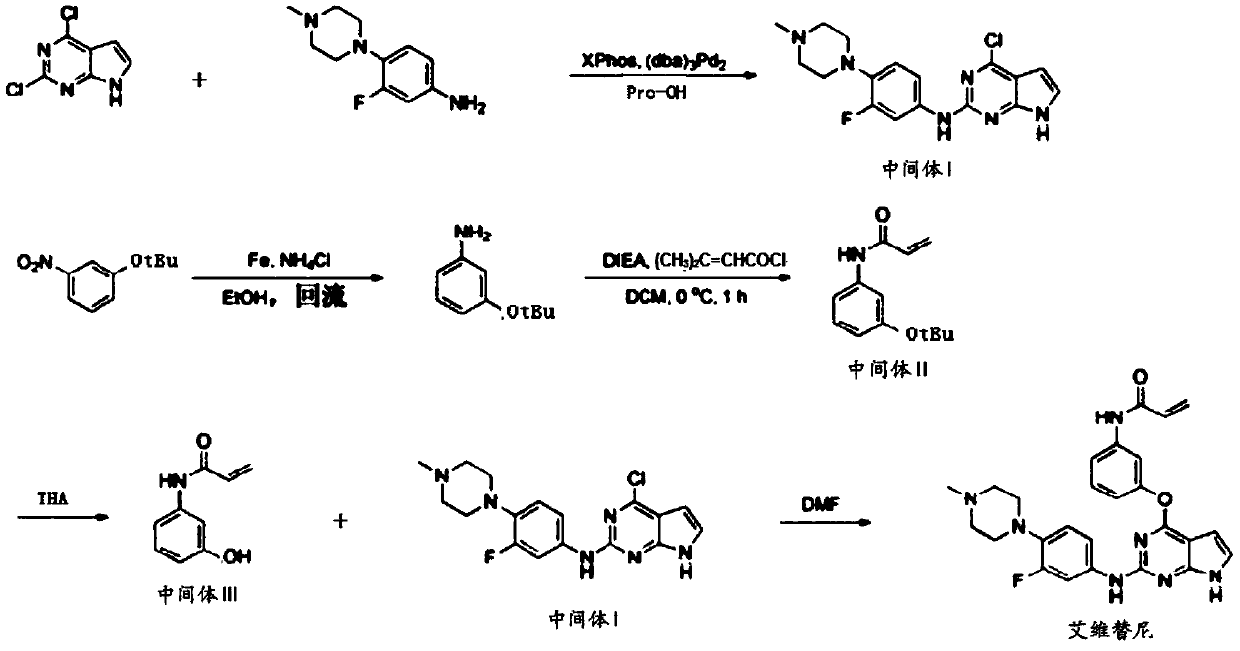
[0020] A preparation method of Avitinib, the reaction scheme is as follows:
[0021]
[0022] Include the following steps:
[0023] A. Combine 2,4-dichloropyrrolopyrimidine with XPhos and Pd 2 (dba) 3 and 3-fluoro-4-(4-methylpiperazin-1-yl)aniline were dissolved in a propanol solvent, and reacted to obtain intermediate I;
[0024] B, the 3-nitrophenol that tBu protects is dissolved in ethanol solvent, add iron powder and ammonium chloride, obtain the 3-aminophenol of tBu protection;
[0025] C. Dissolve tBu-protected 3-aminophenol and diisopropylethylamine in dichloromethane, add isopentenyl chloride dropwise, and react to obtain intermediate II;
[0026] D, intermediate II is refluxed in a trifluoroacetic acid solvent, and tBu is removed to obtain intermediate III;
[0027] E. Mixing and reacting intermediate I, intermediate III and dimethylformamide to obtain a final product. The final product is isolated and purified by preparative HPLC or preparative LC / MS, or by o...
Embodiment 2
[0029] This embodiment is on the basis of embodiment 1:
[0030] In the described A step and E step, all add Na 2 CO 3 Participate in the reaction, and Na 2 CO 3 The added mass is the same as intermediate I. The yield was 72%.
Embodiment 3
[0032] This embodiment is on the basis of embodiment 1:
[0033] In the described A step and E step, all add Na 2 CO 3 Participate in the reaction, and Na 2 CO 3 The added mass is the same as intermediate I.
[0034] In the step A, the reaction was stirred under nitrogen protection at 100°C. The yield was 71%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com