Synthetic method of bazedoxifene and analogues thereof
A synthesis method and technology of bazedoxifene, which are applied in the field of synthesis of bazedoxifene and its analogs, can solve the problems of difficult control of substitution reaction, increase the difficulty of processing, etc., and achieve the advantages of large-scale testing and reduction of reaction steps. , the effect of reducing the cost of synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] The preparation method of the present invention will be further described in detail in conjunction with specific examples below. It should be understood that the following examples are only for illustrating and explaining the present invention, and should not be construed as limiting the protection scope of the present invention. All technologies realized based on the above contents of the present invention are covered within the scope of protection intended by the present invention.
[0062] The experimental methods used in the following examples are conventional methods unless otherwise specified; the reagents and materials used in the following examples can be obtained from commercial sources unless otherwise specified.
Embodiment 1
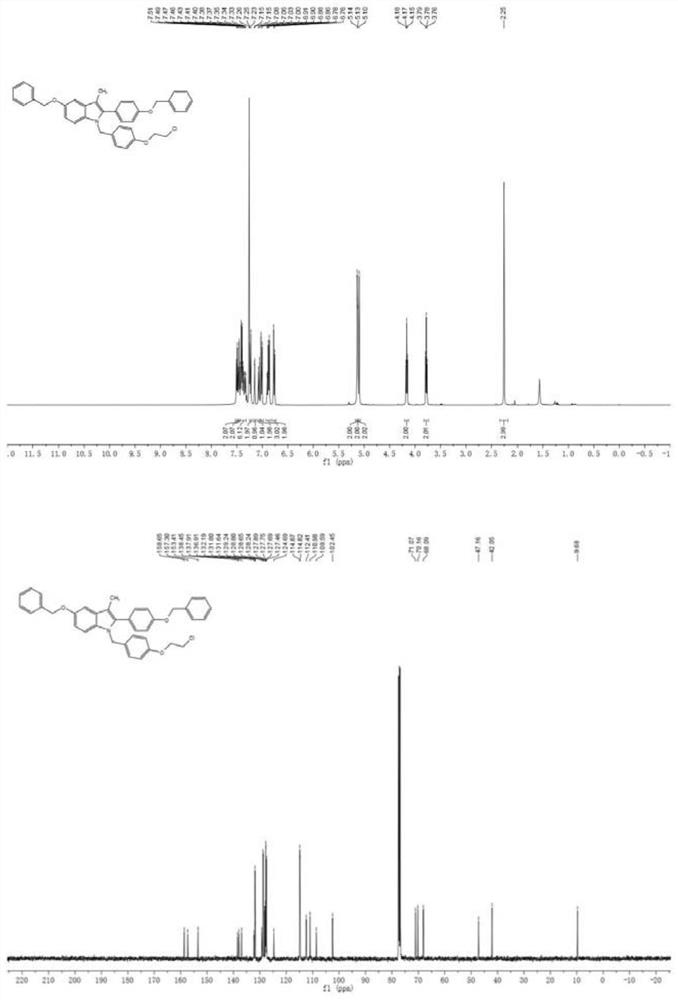
[0064] N-benzyl substituted aniline compound 30 (0.1mmol), methylphenyl alkyne compound 31 (0.15mmol), dichloro(pentamethylcyclopentadienyl) rhodium (trivalent) dimer (2.5 mol%, 0.0016g), AgSbF 6 (10mol%, 0.0035g), pivalic acid (50mol%, 0.0051g), nitrosoisoamyl ester (0.1mmol, 20uL), DCM (1.0mL). 90°C, 24h. After the reaction, it was cooled to room temperature, filtered, rotary evaporated, and used for column chromatography to obtain bazedoxifene precursor compound 32 (47%, purity greater than 95%) as a yellow solid.
[0065]
[0066] Compound 32: 1 H NMR (400MHz, CDCl 3 ): δ7.50(d, J=7.2Hz, 2H), 7.46(d, J=6.6Hz, 2H), 7.44–7.32(m, 6H), 7.24(d, J=8.6Hz, 2H), 7.15 (d, J=2.4Hz, 1H), 7.07(d, J=8.8Hz, 1H), 7.02(d, J=8.6Hz, 2H), 6.93–6.84(m, 3H), 6.77(d, J= 8.7Hz, 2H), 5.14(s, 2H), 5.13(s, 2H), 5.10(s, 2H), 4.17(t, J=5.9Hz, 2H), 3.78(t, J=5.9Hz, 2H) ,2.25(s,3H). 13 C NMR (100MHz, CDCl 3 ): δ158.7, 157.3, 153.4, 138.5, 137.9, 136.9, 132.2, 131.8, 131.6, 129.2, 128.8, 128.7...
Embodiment 2
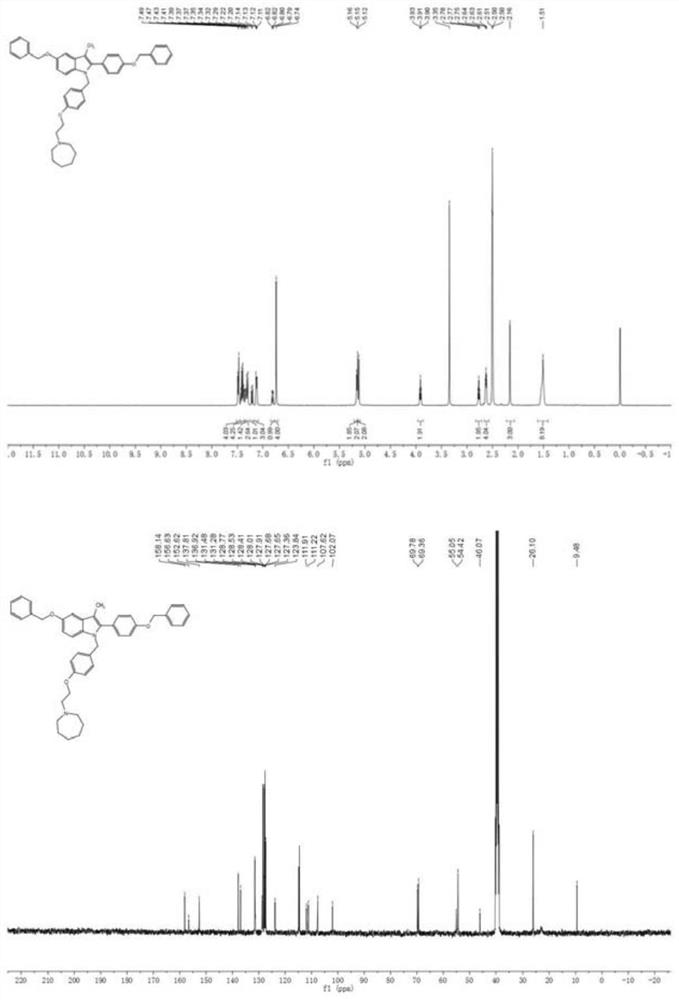
[0068] Bazedoxifene precursor compound 32 (0.2mmol) and hexamethyleneimine 16 (0.4mmol) were refluxed in an acetonitrile solution, a nucleophilic substitution reaction occurred to generate bazedoxifene precursor compound 7 (92%);
[0069]
[0070] Compound 7: 1 H NMR (400MHz, CDCl 3 ): δ7.48-7.46(m,4H),7.45–7.37(m,4H),7.37–7.32(m,2H),7.30(d,J=8.8Hz,2H),7.21(d,J=8.8 Hz, 1H), 7.13(m, 3H), 6.81(dd, J=8.8, 2.4Hz, 1H), 6.74(s, 4H), 5.16(s, 2H), 5.15(s, 2H), 5.12(s ,2H),3.91(t,J=6.1Hz,2H),2.77(t,J=6.0Hz,2H),2.63(m,4H),2.16(s,3H),1.58–1.46(m,8H) . 13 C NMR (100MHz, CDCl 3 ): δ158.1,156.6,152.6,137.8,136.9,131.5,131.3,128.8,128.5,128.4,128.0,127.9,127.7,127.6,127.4,123.8,111.9,111.2,105.6,102.16,6,499. 46.1, 3.10, 9.5. HRMS (ESI) theoretical value C 44 h 47 N 2 o 3 ([M+H]+): 651.3581, measured value: 651.3582.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com