Oncolytic virus and neoantigen combined tumor vaccine and preparation method thereof
A technology of oncolytic virus and tumor vaccine, which is applied in the field of genetic engineering to achieve the effect of improving efficiency and enhancing anti-cancer efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
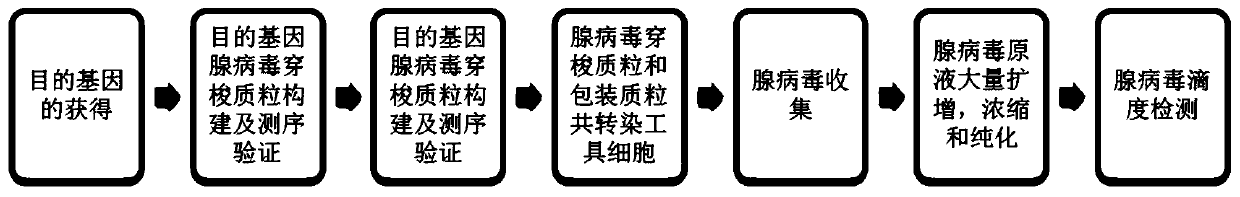
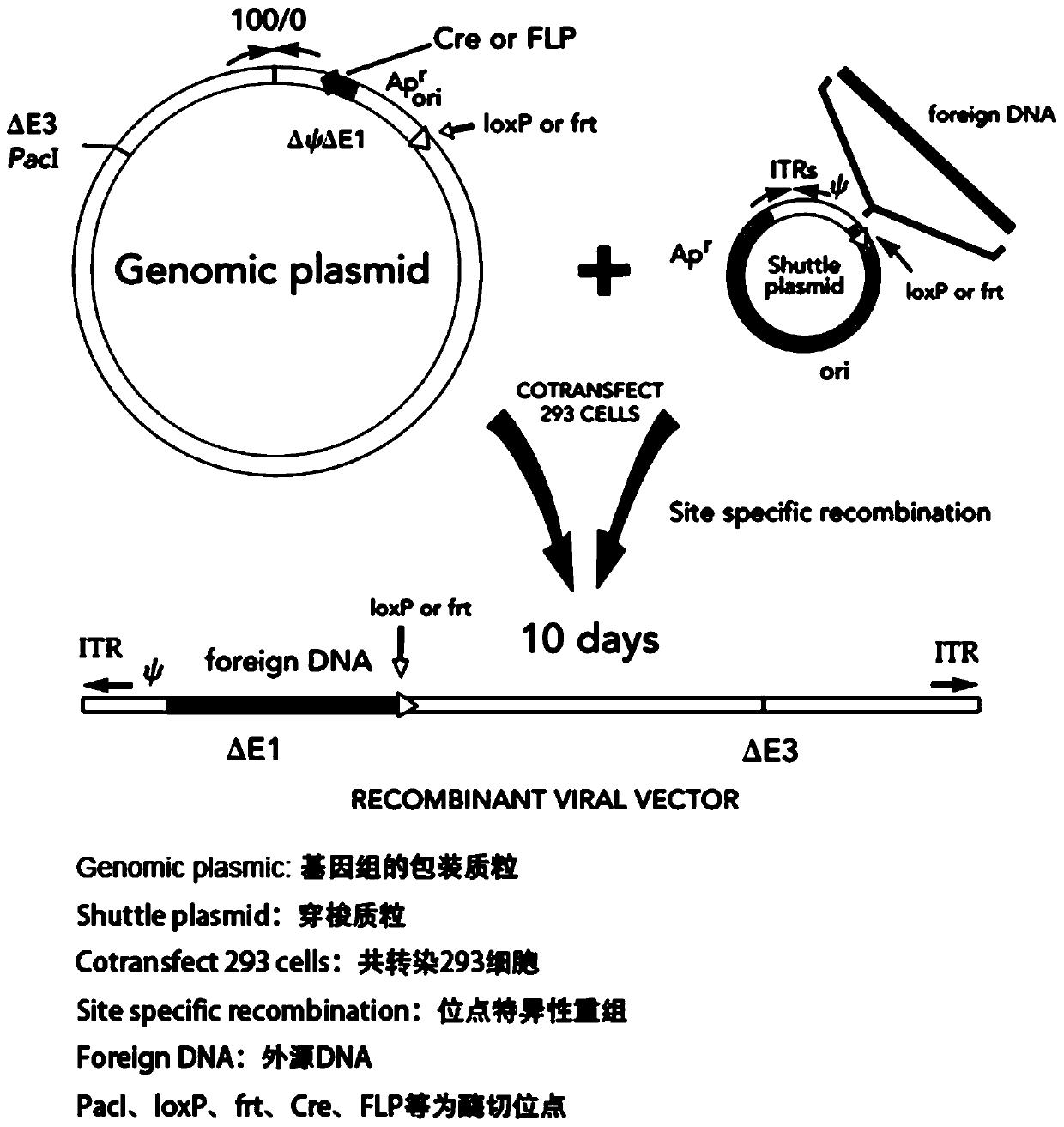
[0048] Embodiment one: sample construction and preparation;
[0049] It mainly includes 4 sample construction and preparation, and the preparation process includes the following contents:
[0050] 1. Identification and preparation of neoantigen polypeptides
[0051] 1.1 Identification of individualized tumor neoantigen polypeptide sites
[0052] The DNA sequencing results of normal cells of C57BL6 mice / tumor patients and the DNA sequencing results of 6 sequencing samples of B16F10 cells / patient tumor cells and inoculated tumors / patient tumors were compared to the mouse / human reference genome from A somatic mutation in the tumor cells is identified in the comparison;
[0053] RNA sequencing was then performed on the three sequencing samples to identify and assess the expression of the mutated allele;
[0054] Predict the type I and type II HLA types of C57BL6 mice / clinical patients based on the genome comparison results, and use the affinity prediction algorithm netMHCpan to...
Embodiment 2
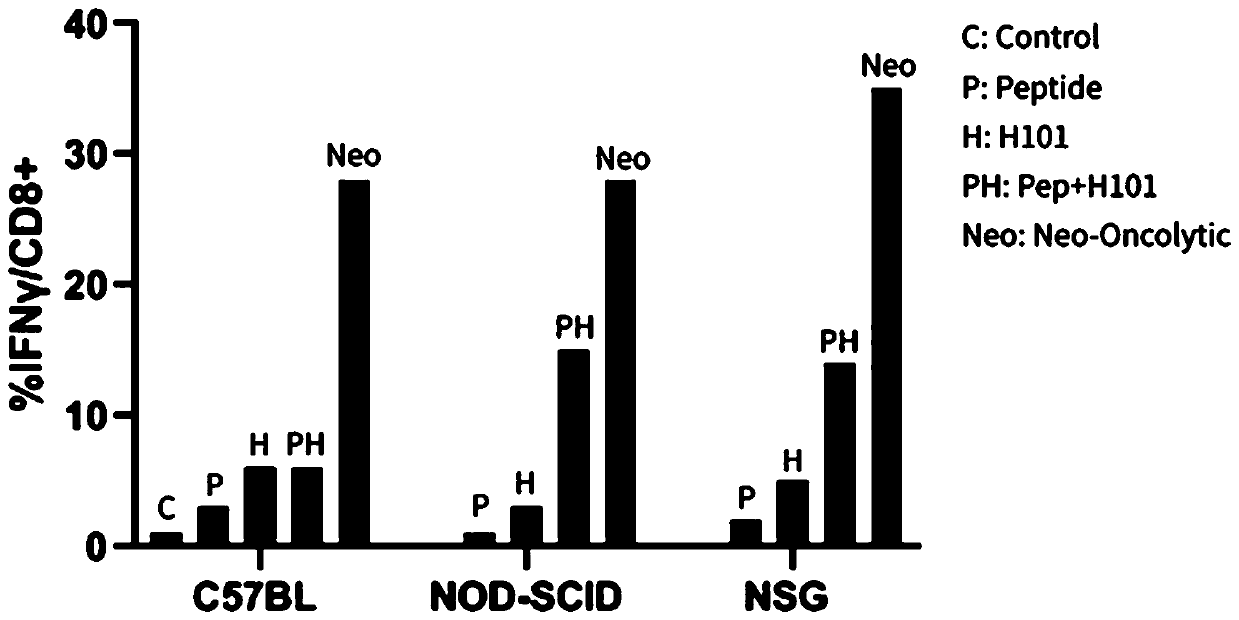
[0079] Example 2: Evaluating the drug efficacy of the combination of oncolytic virus and neoantigen polypeptide vaccine with a mouse-derived pharmacodynamic model;
[0080] 1. Construction of mouse tumor model——C57-B16F10 melanoma model;
[0081] Select C57BL / 6 mice, purchased from Beijing Weitong Lihua, female, 6-8 weeks old. B16-F10 murine melanoma tumor cells were inoculated. Tumor cells were counted before inoculation to ensure that the cell viability was above 95%. The harvested B16-F10 melanoma cells were divided into 7×10 4 cells / Subcutaneous injection in the back only.
[0082] 2. Evaluation of anti-tumor effect
[0083] 2.1 Tumor model grouping;
[0084] Two days after the tumor formation of the mice, 50-60 mice with similar tumor volume and an average tumor diameter of about 0.5 cm were selected and randomly divided into four groups with at least 10 mice in each group, which were the negative control adjuvant group , polypeptide group, oncolytic virus group, po...
Embodiment 3
[0105] Example 3: Drug efficacy evaluation of the combination of oncolytic virus and neoantigen polypeptide vaccine on the humanized drug efficacy model;
[0106] 1. Human-derived tumor model construction
[0107] 1.1 Immune reconstitution humanized PDX mouse model construction - Humanized NOD / SCID mouse PDX model
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com