Method for preparing 2-(2,4-dihydroxyphenyl)-4,6-bisaryl-1,3,5-triazine
A technology of alkyl imidazole tetrafluoroborate and alkyl pyridine tetrafluoroborate, applied in the field of preparing 2--4, can solve problems such as yield restriction, inability to recover and apply aluminum trichloride, and reduce pollution , the recycling process is simple and easy to implement, and the effect of saving catalyst costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
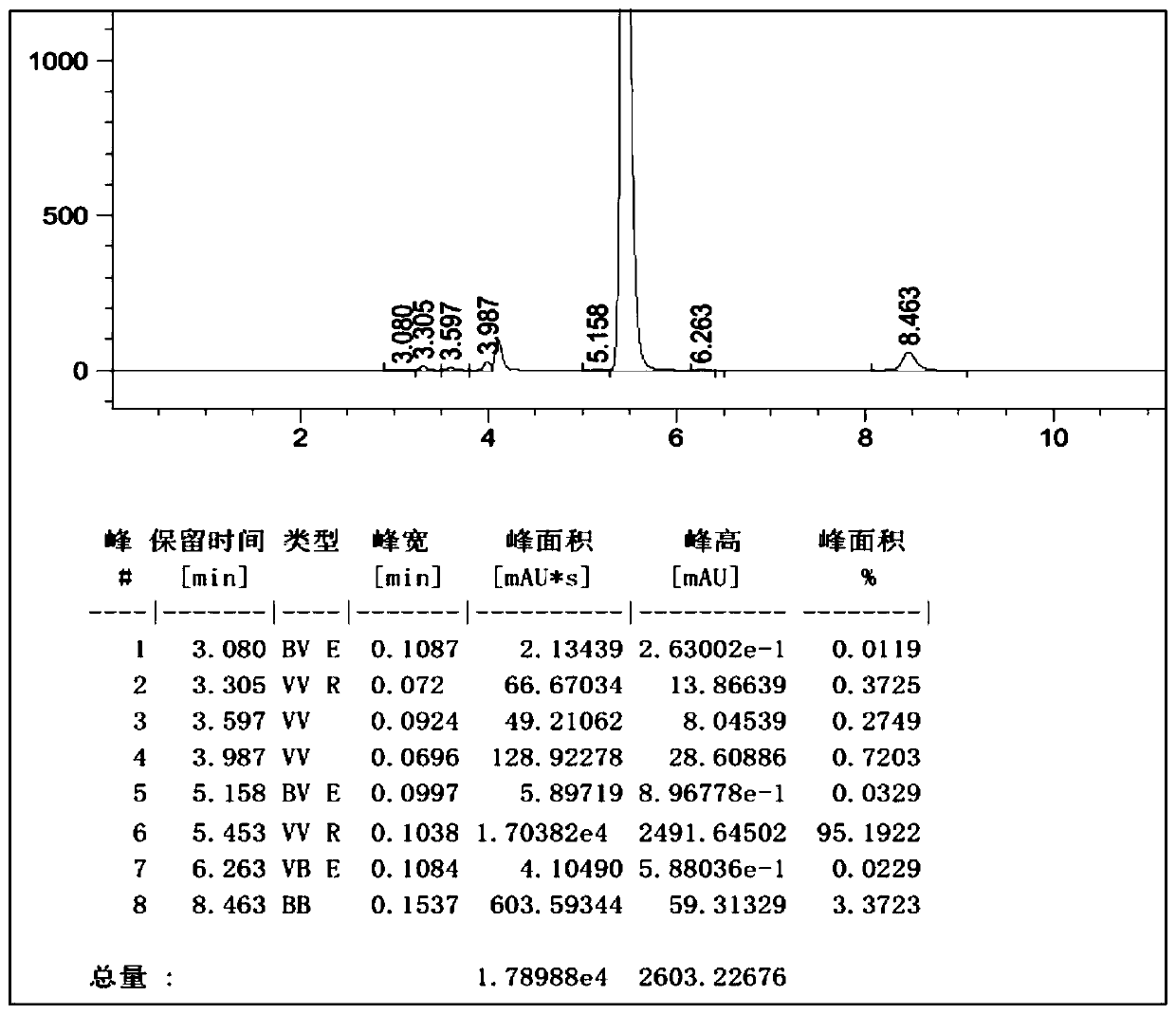
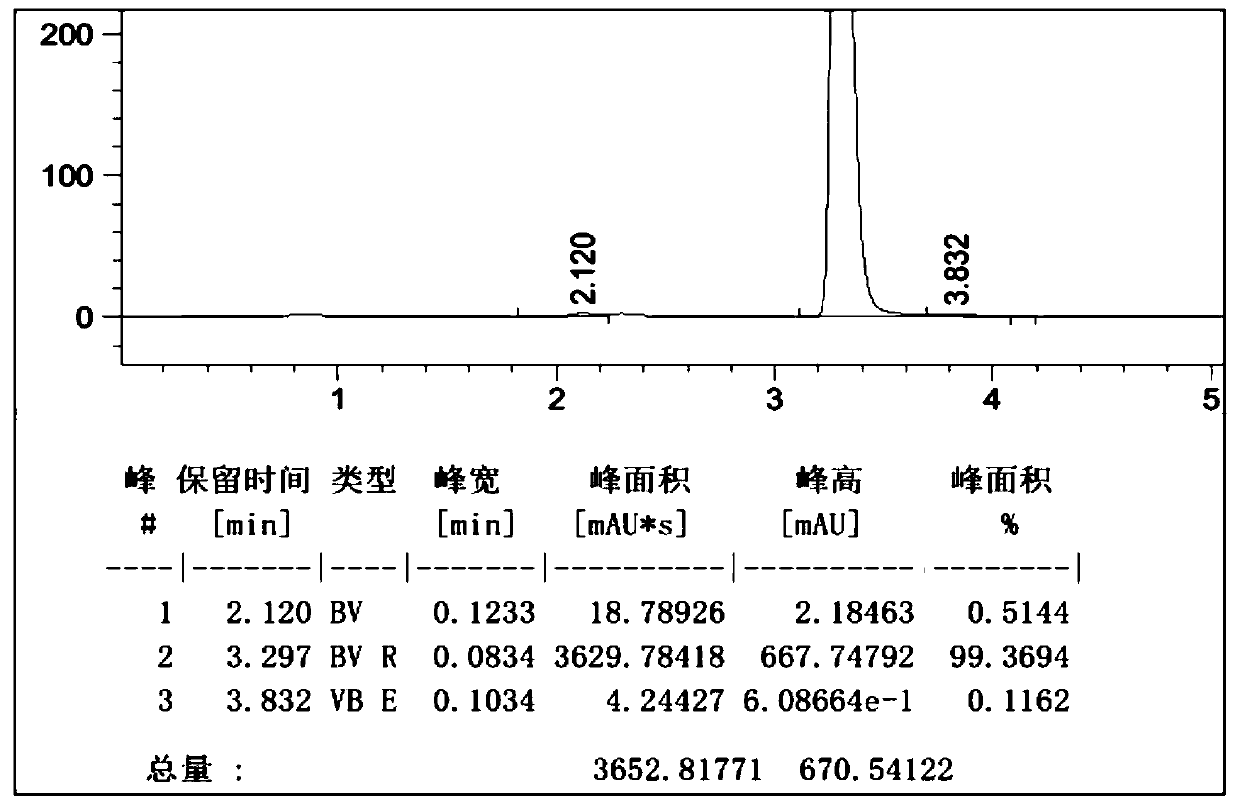
[0050]Example 1: Add 550ml of 1-butyl-3-methylimidazolium tetrafluoroborate, 55ml of o-dichlorobenzene, 55.4g (0.30mol) of cyanuric chloride into the reactor, stir and cool down to 5°C, 46.8g (0.60mol) of benzene was added dropwise, and the dropwise addition time was 2h. While adding benzene dropwise, 3.2g of initiator HCl gas was introduced (completed within 1h), and the temperature was kept at 5-10°C. ~15°C heat preservation reaction for 12 hours, HPLC detection of 2-chloro-4,6-diphenyl-1,3,5-triazine content in the reaction solution was 95.2%, the HPLC spectrum is as follows figure 1 As shown, the peak elution time is consistent with the peak elution time of the standard product; add 34.2g (0.31mol) of resorcinol, stir and heat up to 70-80°C, keep the temperature for 12h, cool down to 20°C to filter out the crude product, and recover the filtrate Apply mechanically, the crude product is refined with methanol to obtain 2-(2,4-dihydroxyphenyl)-4,6-bisphenyl-1,3,5-triazine con...
Embodiment 2
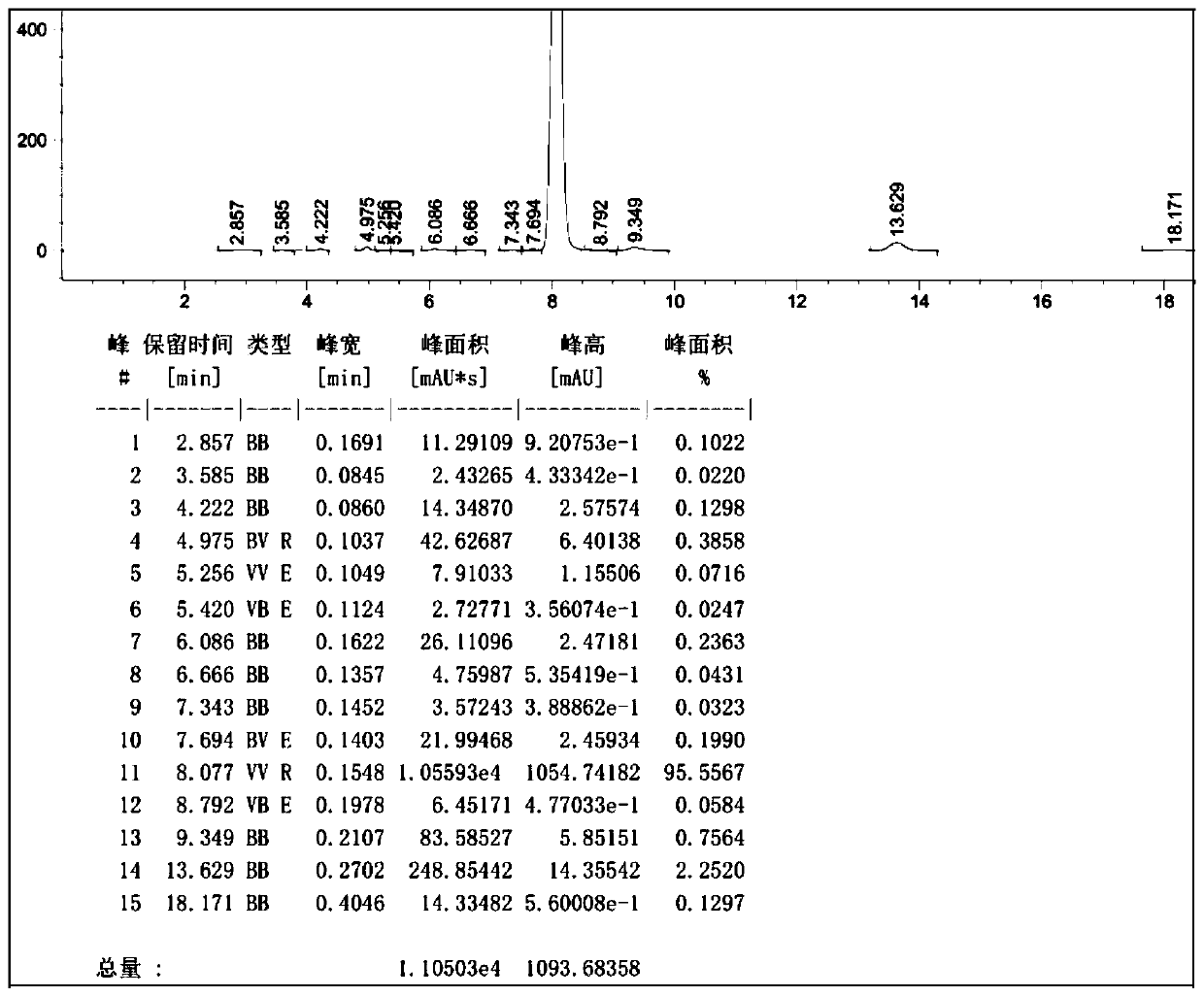
[0051] Example 2: Add 200ml of 1-hexyl-3-methylimidazolium tetrafluoroborate and 100ml of 1-butyl-3-methylimidazolium tetrafluoroborate to the reactor, 55.4g (0.30mol) With cyanuric chloride, stir and cool down to 5°C, add dropwise 46.8g (0.60mol) of benzene, and the dropwise addition time is 3h, while adding dropwise benzene, feed 2.2g of initiator HCl gas (pass through within 1h), keep the temperature at 5~10°C, after the addition of benzene, keep it warm at 10~15°C for 6 hours, HPLC detects that the content of 2-chloro-4,6-diphenyl-1,3,5-triazine in the reaction solution is 94.6%, add m-benzene Diphenol 34.2g (0.31mol), stir and heat up to 90-100°C, heat preservation reaction for 8h, cool down to 20°C to filter out the crude product, recover the filtrate for mechanical use, and refine the crude product with methanol to obtain 2-(2,4-dihydroxyphenyl) - 4,6-bisphenyl-1,3,5-triazine content of 99.2% product, yield 90.5%.
Embodiment 3
[0052] Example 3: Add 200ml of 1-pentyl-3-methylimidazolium tetrafluoroborate and 220ml of 1-butyl-3-methylimidazolium tetrafluoroborate to the reactor, 110ml o-dichlorobenzene , 55.4g (0.30mol) cyanuric chloride, stirring to cool down to 5 ℃, dropwise adding 46.8g (0.60mol) of benzene, the dropping time is 3h, while adding benzene, feed 2.7g of initiator HCl gas (1.5h After passing through), keep the temperature at 5-10°C, and keep the temperature at 10-15°C for 9h after the addition of benzene, and detect 2-chloro-4,6-diphenyl-1,3,5-triazine in the reaction solution by HPLC Content 95.0%, add resorcinol 34.2g (0.31mol), stir and heat up to 80-90°C, heat preservation reaction for 10h, cool down to 20°C to filter out the crude product, recover the filtrate for mechanical use, and refine the crude product with methanol to obtain 2-(2, 4-dihydroxyphenyl)-4,6-bisphenyl-1,3,5-triazine with a content of 99.4% and a yield of 91.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com