Construction method of HPLC fingerprint and detection method of Qian Bai Bi Yan Pian
A technology of Qianbo Biyan tablet and fingerprint spectrum, applied in the field of traditional Chinese medicine analysis and detection, can solve the problems of complex chemical composition, inability to control product quality as a whole, difficult to fully reflect the overall quality characteristics of products, etc. The chromatogram baseline is stable and the chromatographic peak separation is good.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
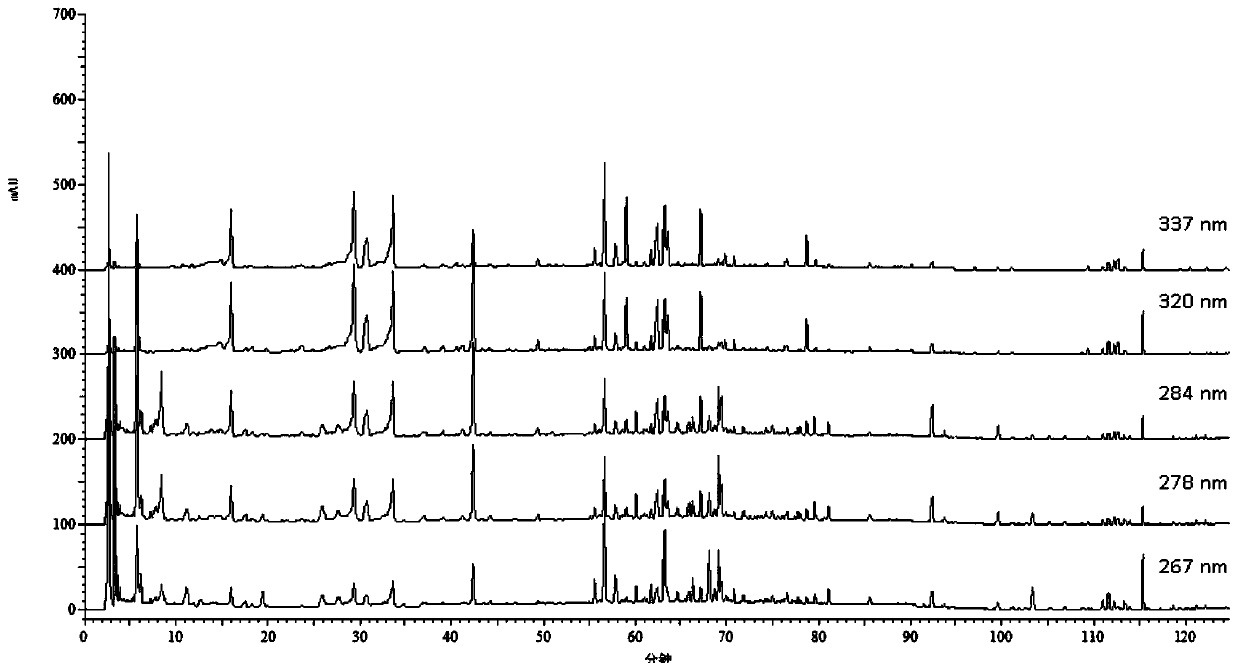
[0072] Construction of Example 1 Qianbai Biyan Tablet HPLC Fingerprint
[0073] 1. Instrument: Agilent 1260 high performance liquid chromatograph, ML204 / 02 analytical balance.
[0074] 2. Reagents: Liquid chromatography analytical reagents are chromatographically pure, other reagents are analytically pure, and water is ultrapure water. Qianbai Biyan Tablets are provided by Guangzhou Baiyunshan Qixing Pharmaceutical Co., Ltd.
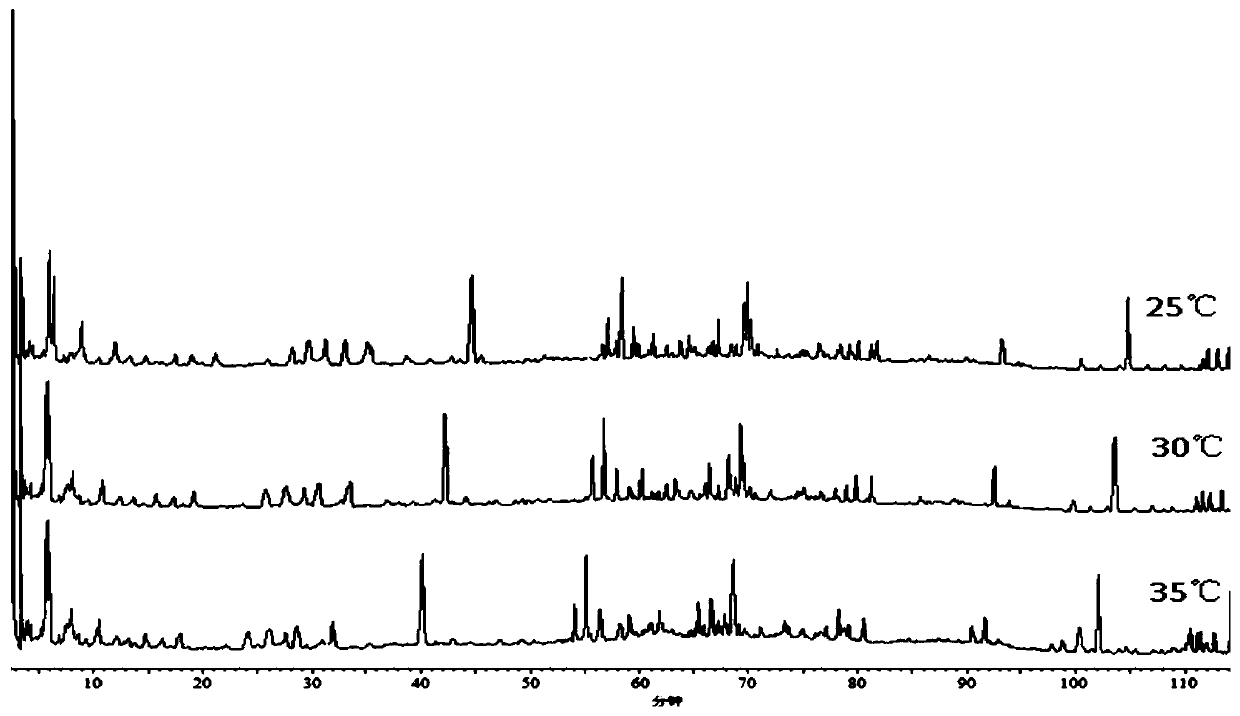
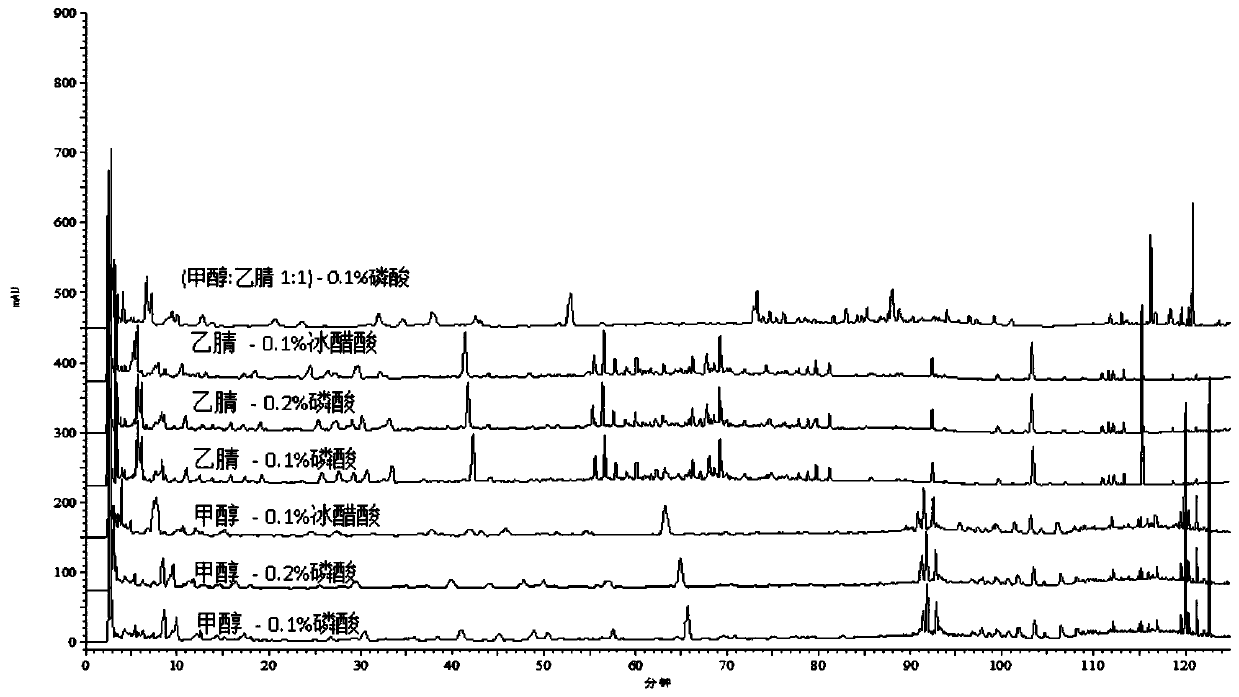
[0075] 3. Methods and results
[0076] 3.1 Mixed reference substance solution: Accurately weigh hyperoside, p-hydroxycinnamic acid, notopterin alcohol, chrysophanol, isoimperatorin, chlorogenic acid, imperatorin, abiflavonoids, emodin methyl ether, Emodin, osthole, caffeic acid, kaempferol reference substance amount, made by adding methanol. Each 1ml solution contains 200μg hyperoside, 100μg p-hydroxycinnamic acid, 60μg notopteryl alcohol, 100μg chrysophanol, 60μg isoimperiana A mixed reference solution of chlorogenic acid, 200 μg chlorogenic acid, 40 ...
Embodiment 2
[0154] The present embodiment provides a kind of detection method of Qianbai Biyan Tablets, the steps are as follows:
[0155] Preparation of the sample to be tested: Take Qianbai Biyan Tablets to be tested (batch numbers: 18001, 18020, 19007, 19008, 19009, provided by Guangzhou Baiyunshan Qixing Pharmaceutical Co., Ltd.), remove the coating from the sugar-coated tablet, grind finely, and accurately Weigh 1.6g, accurately add 25ml methanol, weigh the weight, extract by ultrasonic (power 250W, frequency 80kHz) for 60min, let cool, then weigh again, use the above methanol solvent to make up the lost weight, shake well, and use 0.45μm micrometer Pore membrane filtration, take the continued filtrate, that is.
[0156] Chromatographic conditions: refer to "chromatographic conditions" under item 3.3 of Example 1.
[0157] Precisely draw 10 μl of the sample solution to be tested, inject it into the ultra-high performance liquid chromatograph, and inject the sample according to the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com