Smelly seven secondary metabolites and their preparation methods and their application in pharmacy
A technology for the generation and metabolites of stinky seven times, which is applied in the field of medicine, and can solve the problems of no anti-inflammatory related pharmacological activities, no reports of anti-inflammatory drugs and pharmaceutical preparations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
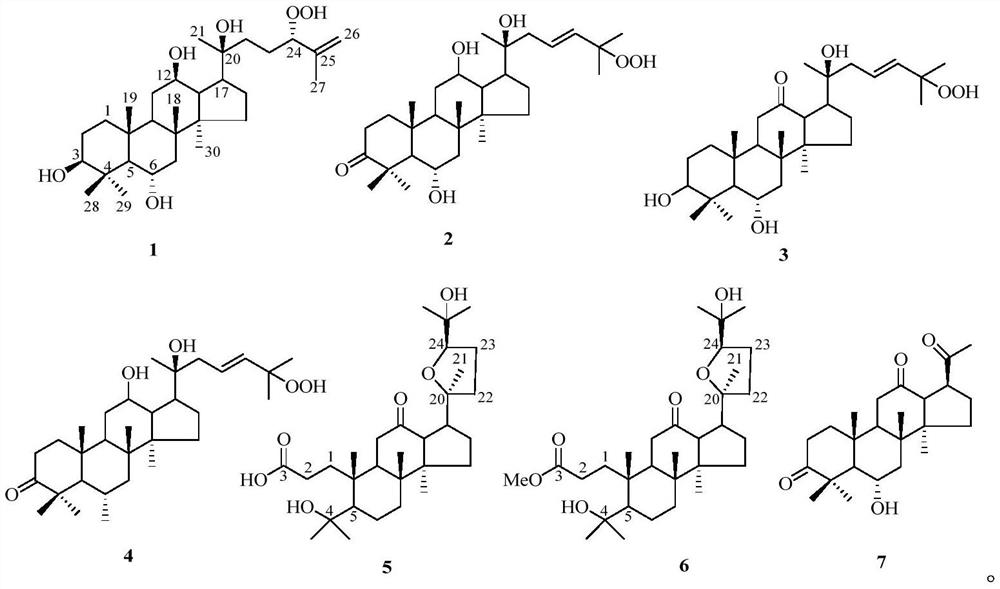
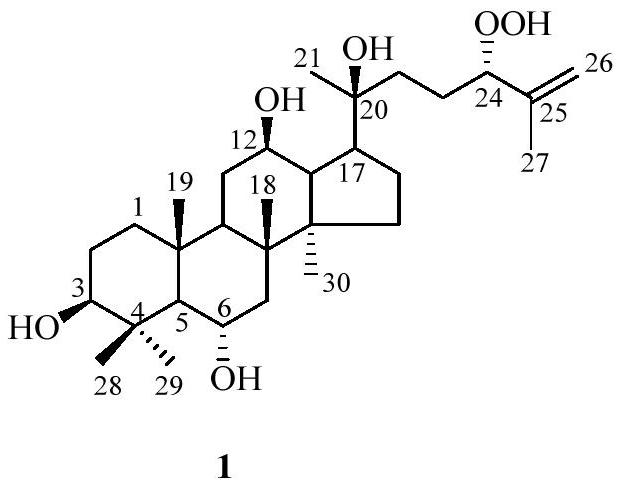
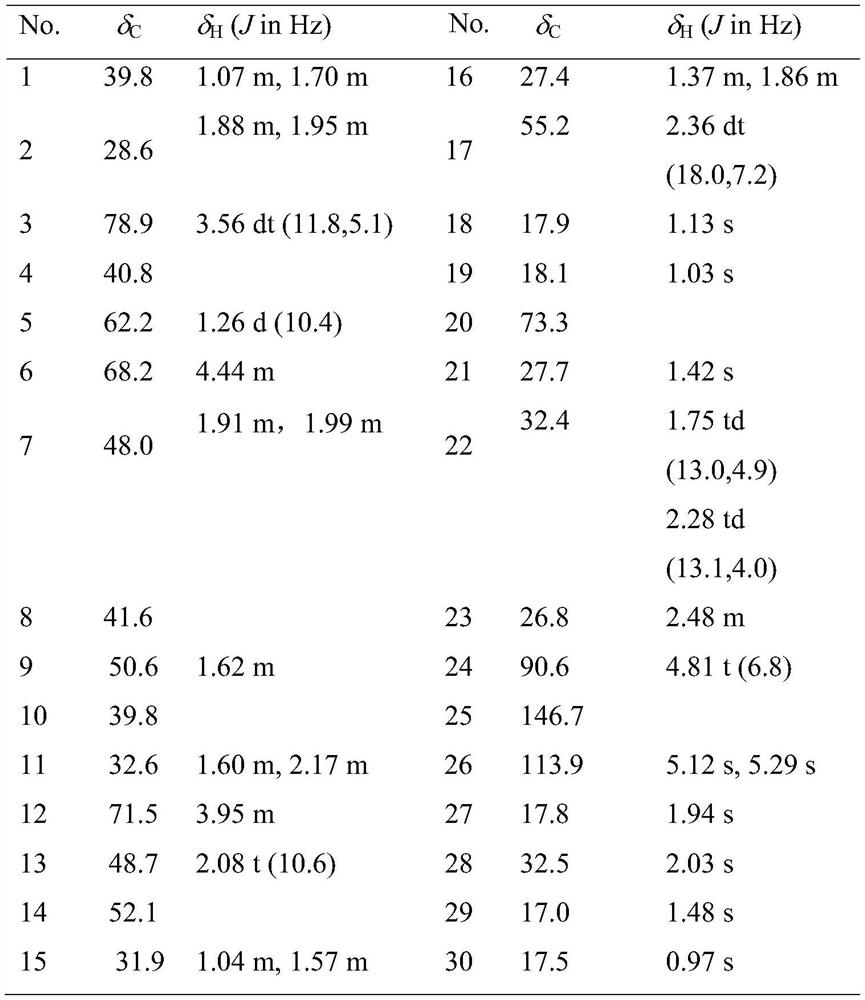
[0021] Preparation of 20(S)-dammar-25-ene-24(S)-hydroperoxyl-3β,6α,12β,20-tetrol(1) and its application in medicine.
[0022]
[0023] Step 1: Preparation of compound 20(S)-dammar-25-ene-24(S)-hydroperoxyl-3β, 6α, 12β, 20-tetrol(1).
[0024] The air-dried stinky seven was pulverized, extracted with methanol under reflux for 3 times at 60°C, and filtered. The filtrate was concentrated in vacuo to remove the organic solvent, applied to a macroporous resin chromatography column, eluted with pure water to remove the polysaccharide, and then eluted with methanol to obtain the crude saponin. The crude saponin was applied to a silica gel chromatography column and eluted with a solvent with a volume ratio of chloroform:methanol of 7:3 to obtain three fractions, namely A, B and C. Part B continued to be applied to RP-18, and gradient elution was carried out with a solvent with a methanol:water volume ratio from 1:9 to 9:1 to obtain 8 parts, namely B1-B8, and part B3 was applied to ...
Embodiment 2
[0033] Preparation of 20(S)-dammar-3-oxo-23-ene-25-hydroperoxyl-6α,12β,20-triol(2) and its application in medicine.
[0034]
[0035] Step 1: Preparation of compound 20(S)-dammar-3-oxo-23-ene-25-hydroperoxyl-6α, 12β, 20-triol(2).
[0036] The air-dried stinky seven was pulverized, extracted with methanol under reflux for 3 times at 60°C, and filtered. The filtrate was concentrated in vacuo to remove the organic solvent, applied to a macroporous resin chromatography column, eluted with pure water to remove the polysaccharide, and then eluted with methanol to obtain the crude saponin. The crude saponin was applied to a silica gel chromatography column and eluted with a solvent with a volume ratio of chloroform:methanol of 7:3 to obtain three fractions, namely A, B and C. Part B was continued on RP-18, and eluted with a solvent with a volume ratio of methanol:water from 1:9 to 9:1 to obtain 8 parts, namely B1-B8, and B1 was continued on silica gel chromatography column with c...
Embodiment 3
[0045] Preparation of 20(S)-dammar-12-oxo-23-ene-25-hydro-peroxyl-3β,6α,20-triol(3) and its application in medicine.
[0046]
[0047] Step 1: Preparation of compound 20(S)-dammar-12-oxo-23-ene-25-hydro-peroxyl-3β,6α,20-triol(3).
[0048] The air-dried stinky seven was pulverized, extracted with methanol under reflux for 3 times at 60°C, and filtered. The filtrate was concentrated in vacuo to remove the organic solvent, applied to a macroporous resin chromatography column, eluted with pure water to remove the polysaccharide, and then eluted with methanol to obtain the crude saponin. The crude saponin was applied to a silica gel chromatography column and eluted with a solvent with a volume ratio of chloroform:methanol of 7:3 to obtain three fractions, namely A, B and C. Part B was continued on RP-18, and eluted with a solvent with a volume ratio of methanol:water from 1:9 to 9:1 to obtain 8 parts, namely B1-B8, and B1 was continued on silica gel chromatography column with c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com