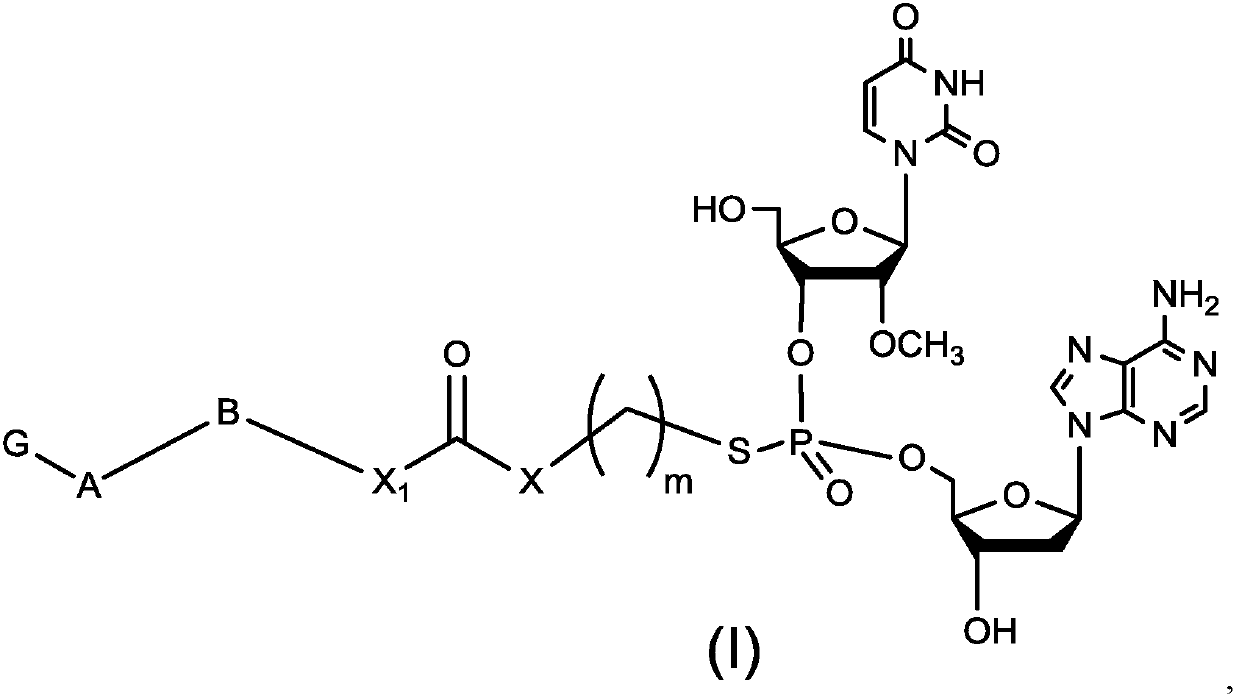
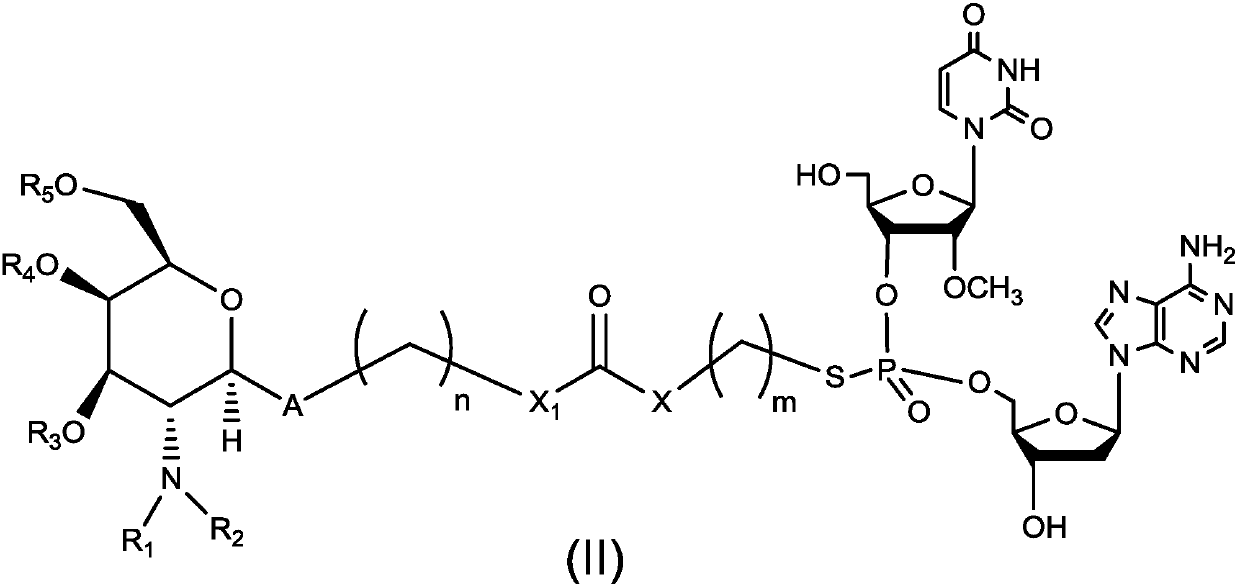
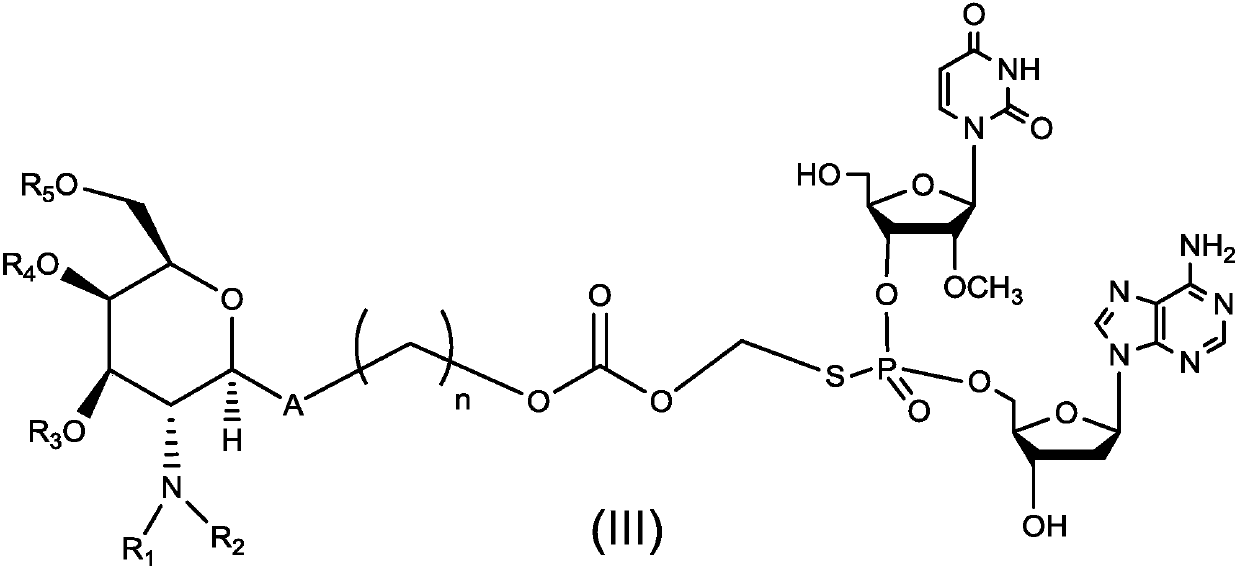
Dinucleotide prodrug
A technology of dinucleosides and precursor compounds, which can be applied to sugar derivatives, pharmaceutical formulations, organic active ingredients, etc., and can solve problems such as not meeting the Lipinski standard
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] The synthetic method of embodiment 1 compound BG001:
[0117]
[0118] Step 1: Preparation of intermediate NB-08
[0119] Add SM1 (50g, 0.1284mol), 500mL of dichloromethane, and Molecular sieve 50g. It was then stirred for 1 h under nitrogen protection. Trimethylsilyl trifluoromethanesulfonate (30ml, 0.1658mol) was slowly added dropwise to the reaction solution under an ice-water bath, and the reaction was refluxed for 40h after the dropwise addition was completed. The reaction solution was brown-black. After TLC monitors that the reaction is complete, it is lowered to room temperature, and triethylamine is added dropwise until neutral. After filtering, the filtrate was extracted and washed with ice water (3×300ml), and the aqueous layer was discarded. The organic layer was dried over anhydrous sodium sulfate, stirred for 1 h, and filtered. The filtrate was stirred and dried with anhydrous sodium sulfate for 1-2 hours. Filter to obtain a dichloromethane solut...
Embodiment 2
[0145] Embodiment 2: Compound BG002 synthetic method
[0146]
[0147] Step 1: Preparation of intermediate NB-08
[0148] Add SM1 (50g, 0.1284mol), 500mL of dichloromethane, and Molecular sieve 50g. It was then stirred for 1 h under nitrogen protection. Trimethylsilyl trifluoromethanesulfonate (30ml, 0.1658mol) was slowly added dropwise to the reaction solution under an ice-water bath, and the reaction was refluxed for 40h after the dropwise addition was completed. The reaction solution was brown-black. After TLC monitors that the reaction is complete, it is lowered to room temperature, and triethylamine is added dropwise until neutral. After filtering, the filtrate was extracted and washed with ice water (3×300ml), and the aqueous layer was discarded. The organic layer was dried over anhydrous sodium sulfate, stirred for 1 h, and filtered. The filtrate was stirred and dried with anhydrous sodium sulfate for 1-2 hours. Filter to obtain a dichloromethane solution of N...
Embodiment 3
[0169] The preparation of embodiment 3 compound BG003
[0170]
[0171]
[0172] Step 1: Preparation of intermediate NB-08
[0173] Add SM1 (50g, 0.1284mol), 500mL of dichloromethane, and Molecular sieve 50g. It was then stirred for 1 h under nitrogen protection. Trimethylsilyl trifluoromethanesulfonate (30ml, 0.1658mol) was slowly added dropwise to the reaction solution under an ice-water bath, and the reaction was refluxed for 40h after the dropwise addition was completed. The reaction solution was brown-black. After TLC monitors that the reaction is complete, it is lowered to room temperature, and triethylamine is added dropwise until neutral. After filtering, the filtrate was extracted and washed with ice water (3×300ml), and the aqueous layer was discarded. The organic layer was dried over anhydrous sodium sulfate, stirred for 1 h, and filtered. The filtrate was stirred and dried with anhydrous sodium sulfate for 1-2 hours. Filter to obtain a dichloromethane...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com