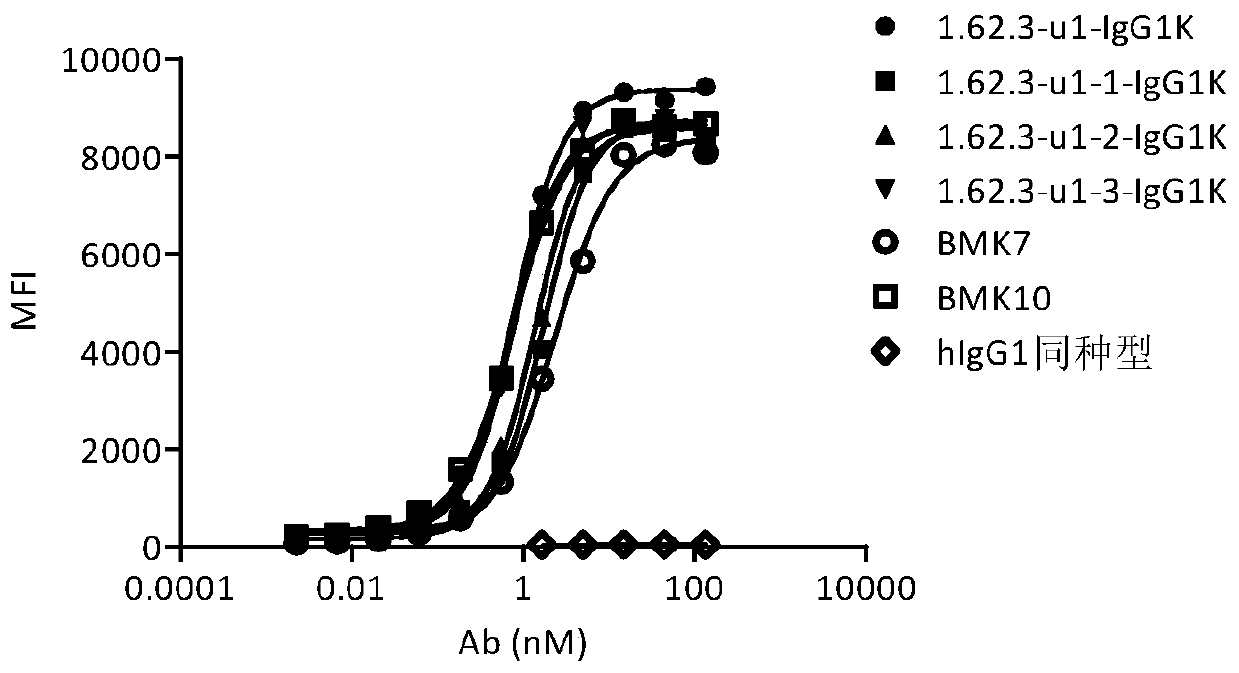
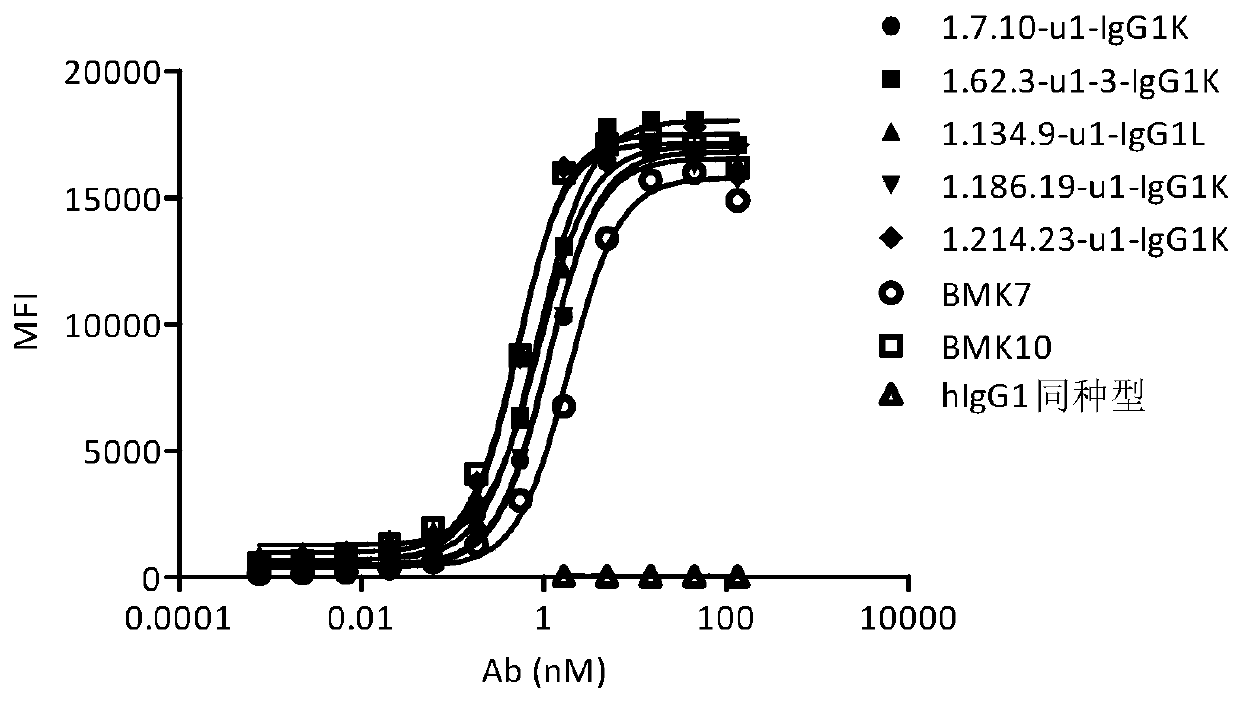
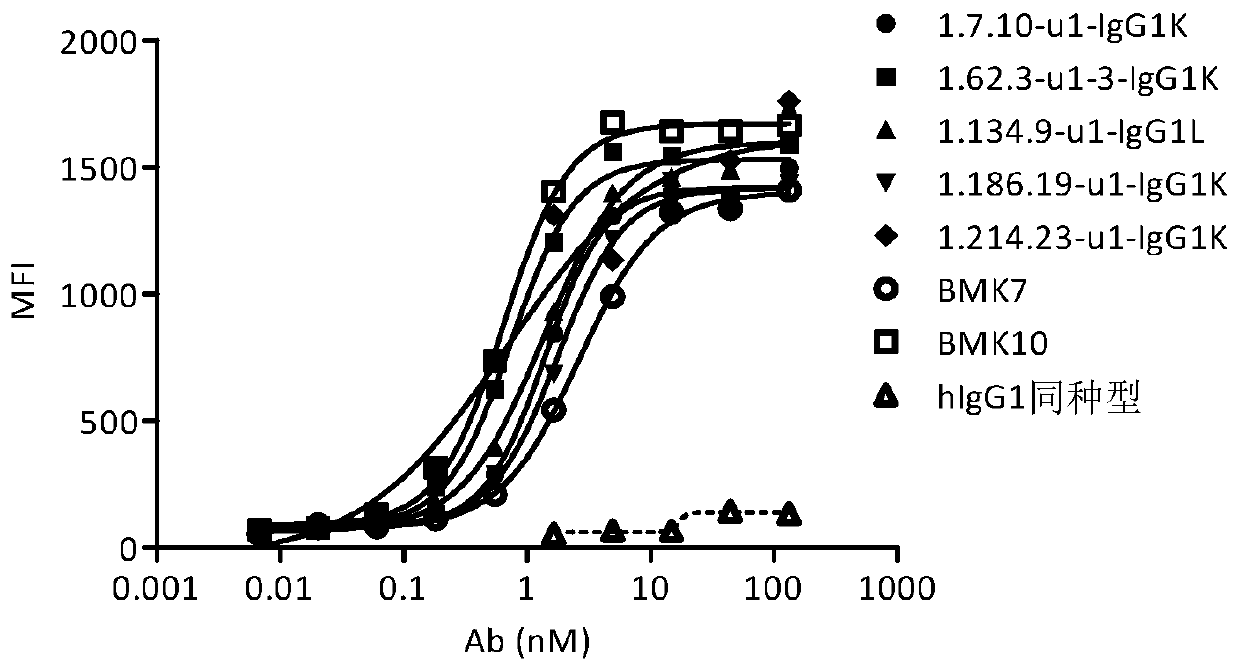
Anti-OX40 fully human antibody and preparation method and application thereof
An antibody and whole-human technology, applied in botany equipment and methods, biochemical equipment and methods, antibodies, etc., can solve the problems of high immunogenicity reduction, toxicity, and limited clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0428] material preparation
[0429] 1.1 Production of immunogen
[0430] A cDNA encoding the extracellular domain (ECD) of the OX40 protein was synthesized by Sangon Biotech (GenBank refCAB96543.1) and inserted into a modified expression vector pcDNA3.3 (ThermoFisher). Plasmid DNA is prepared on a large scale and the inserted DNA sequence is verified by sequencing. The fusion protein OX40ECD coupled with human Fc or His tag was obtained by transfecting human OX40ECD gene into Freestyle 293F (ThermoFisher) or Expi-293F cells (ThermoFisher). After 5 days, supernatants were harvested from transiently transfected cell cultures. Fusion proteins are purified and quantified for immunization and screening.
[0431] 1.2 Production of reference antibody
[0432] Four reference antibodies, BMK1, BMK5, BMK7 and BMK10, were used as positive controls in the examples. BMK1 was synthesized according to the cloning of 11D4 of US Patent No. US8236930B2 (Pfizer). BMK5 was synthesized acco...
Embodiment 2
[0436] Production of Antibody Hybridomas
[0437] 2.1 Immunization and cell fusion
[0438] OMT rats were used to generate fully human monoclonal antibodies against OX40 comprising chimeric polynucleotides useful for optimal production of functional immunoglobulins with a human idiotype. This rat strain carries human heavy and light chain transgenes as described in PCT Publication WO2014 / 093908. In order to produce a fully human monoclonal antibody against OX40, use aluminum phosphate (Alum-Phos) as an adjuvant to mix 20 μg human OX40 ECD protein to immunize OMT rats aged 6-8 weeks at the footpad, and mix TiterMax with 20 μg human OX40 ECD protein was injected subcutaneously for the first booster and repeated every two weeks with Alum-Phos and TiterMax mixed human OX40ECD protein. Serum antibody titers were measured every 1 or 2 weeks by enzyme-linked immunosorbent assay (ELISA). When the serum antibody titer was high enough, rats were given a final booster immunization wit...
Embodiment 3
[0445] Construction and purification of fully human antibody molecules
[0446] 3.1 Hybridoma sequencing
[0447] RNA was extracted from monoclonal hybridoma cells using RNeasy Plus Mini Kit (Qiagen) and Trizol reagent. The heavy chain variable region (VH) and light chain variable region (VL) of the OX40 chimeric antibody were amplified as follows. Briefly, RNA is first reverse transcribed into cDNA using reverse transcriptase, as described below.
[0448] Table 1. cDNA amplification reaction (20μL)
[0449]
[0450] Table 2 cDNA amplification reaction conditions
[0451] step 1 step 2 step 3 step 4 temperature 25 50 85 4 time 10min 50min 5min ∞
[0452] The resulting cDNA is used as a template for subsequent PCR amplification using primers specific for the gene of interest. The PCR reaction was performed as follows.
[0453] Table 3. PCR reaction system (50μL)
[0454] components Dosage cDNA 2.0 μL P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com