A method for splitting propranolol using menthol-lactic acid hydrophobic deep eutectic solvent
A technology of deep eutectic solvent and propranolol hydrochloride, which is applied in the field of drug preparation, can solve problems such as general splitting effect, and achieve the effects of reducing harm to users and the environment, low cost, and low difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
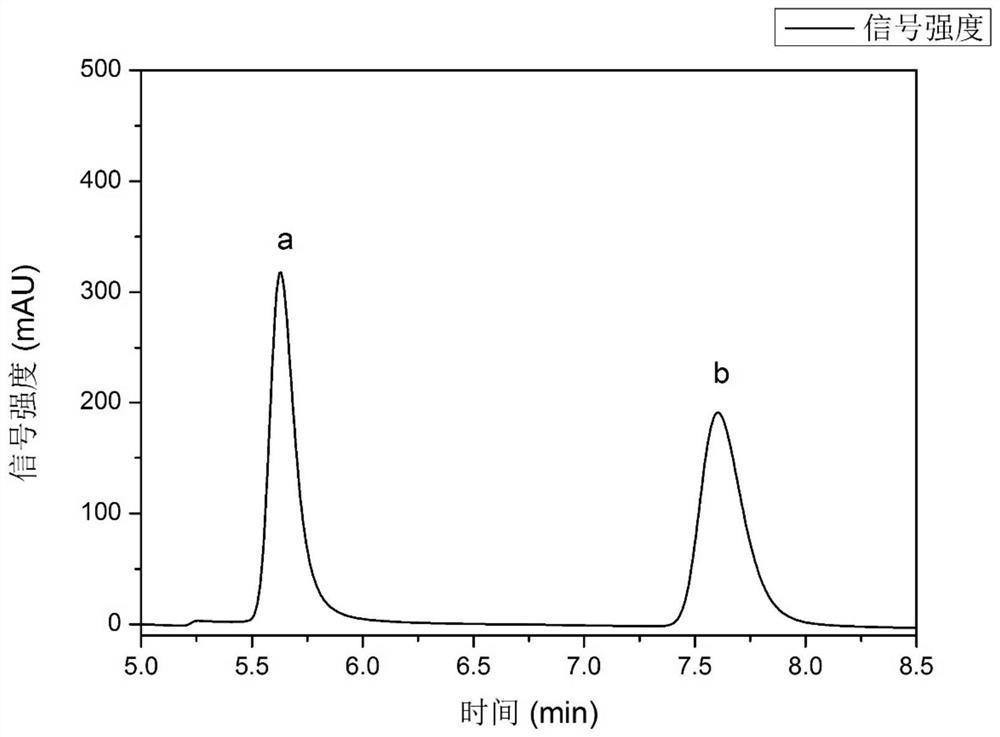
Embodiment 1
[0049] A method for splitting propranolol using a menthol-lactic acid hydrophobic deep eutectic solvent, comprising the following steps:
[0050] (1) Put (-)-menthol and L(+)-lactic acid in a round-bottomed flask with a ratio of 1:1 and mix them evenly, and carry out the first heat treatment at a stirring state with a rotation speed of 800rpm, The temperature of the first heat treatment is 80°C, and the time of the first heat treatment is 1h, and a clear and transparent solution is obtained, then the stirring is stopped, and the heat preservation treatment is carried out at 80°C for 0.5h, and cooled to room temperature to obtain a hydrophobic low ethanol Solvent (-)-menthol-L(+)-lactic acid;
[0051] (2) 500mg of propranolol hydrochloride is added in 5mL alkaline solution (the NaOH solution that selects concentration to be 1.5mol / L here), in the stirring state that rotating speed is 800rpm, carry out heat treatment for the second time, heat for the second time The temperature...
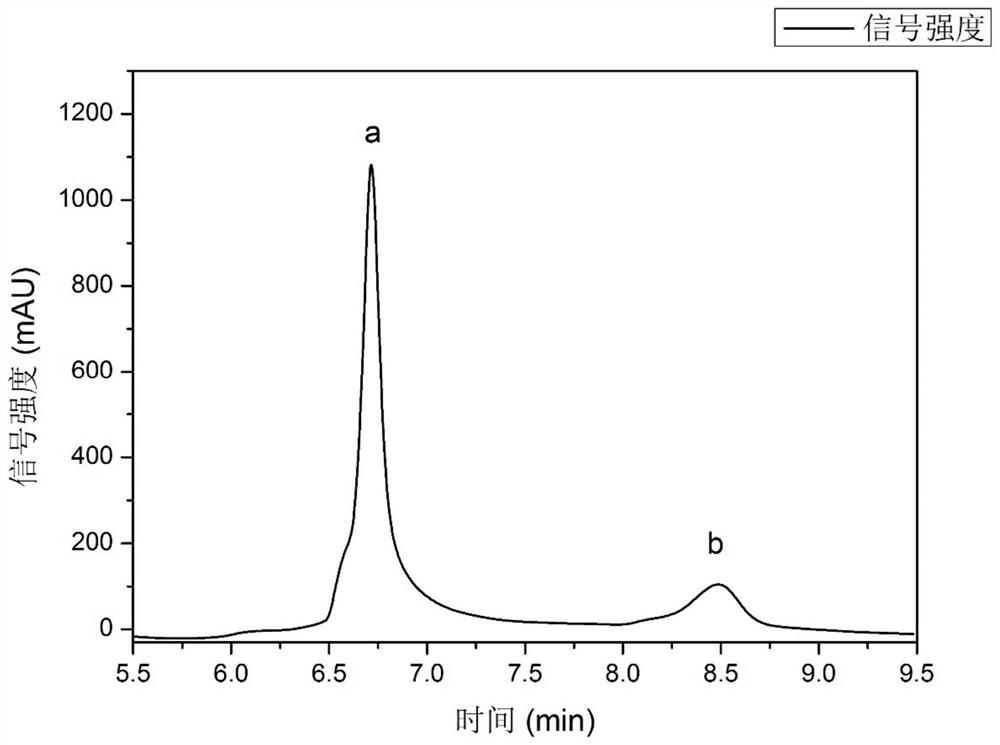
Embodiment 2
[0061] A method for splitting propranolol using a menthol-lactic acid hydrophobic deep eutectic solvent, comprising the following steps:
[0062] (1) Put (-)-menthol and L(+)-lactic acid with the amount of 1:1 in a round-bottomed flask and mix evenly, and carry out the first heat treatment under the stirring state with a rotation speed of 600rpm. The temperature of the second heat treatment is 80°C, and the time of the first heat treatment is 1.5h to obtain a clear and transparent solution, then stop stirring, carry out heat preservation treatment at 80°C for 2h, and cool to room temperature to obtain a hydrophobic deep eutectic solvent (-)-menthol-L(+)-lactic acid;
[0063] (2) 500mg of propranolol hydrochloride is added in 5.5mL alkaline solution (selection concentration is the NaOH solution of 1.5mol / L here), in the stirring state that rotating speed is 600rpm, carry out heat treatment for the second time, the second time The heat treatment temperature is 45°C, the second ...
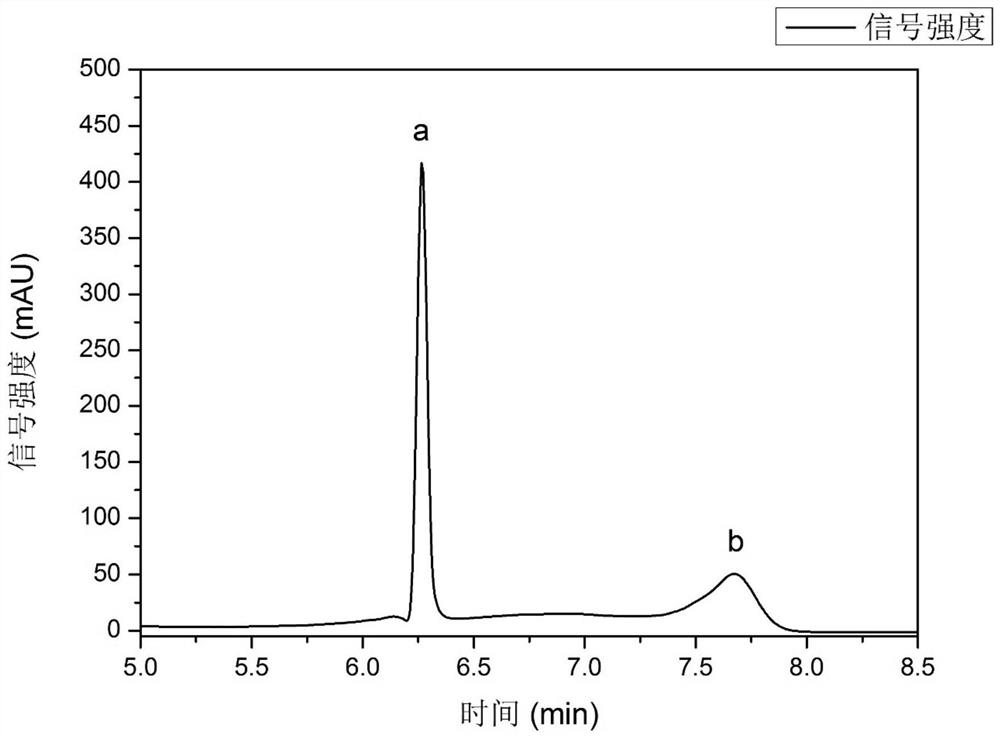
Embodiment 3
[0068] A method for splitting propranolol using a menthol-lactic acid hydrophobic deep eutectic solvent, comprising the following steps:
[0069] (1) Place (-)-menthol and L(+)-lactic acid in a round-bottomed flask with a ratio of 1:1.3 and mix them uniformly, and carry out the first heat treatment at a stirring state of 500 rpm. The temperature of the first heat treatment is 60°C, and the time of the first heat treatment is 1.5h to obtain a clear and transparent solution, then stop stirring, carry out heat preservation treatment at 60°C for 0.5h, and cool to room temperature to obtain a low hydrophobicity solution. Eutectic solvent (-)-menthol-L(+)-lactic acid;
[0070] (2) 500mg propranolol hydrochloride is added in 5mL alkaline solution (concentration is the KOH solution of 2mol / L for selection here), in the stirring state that rotating speed is 500rpm, carry out heat treatment for the second time, heat treatment for the second time The temperature is 35°C, the time of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com