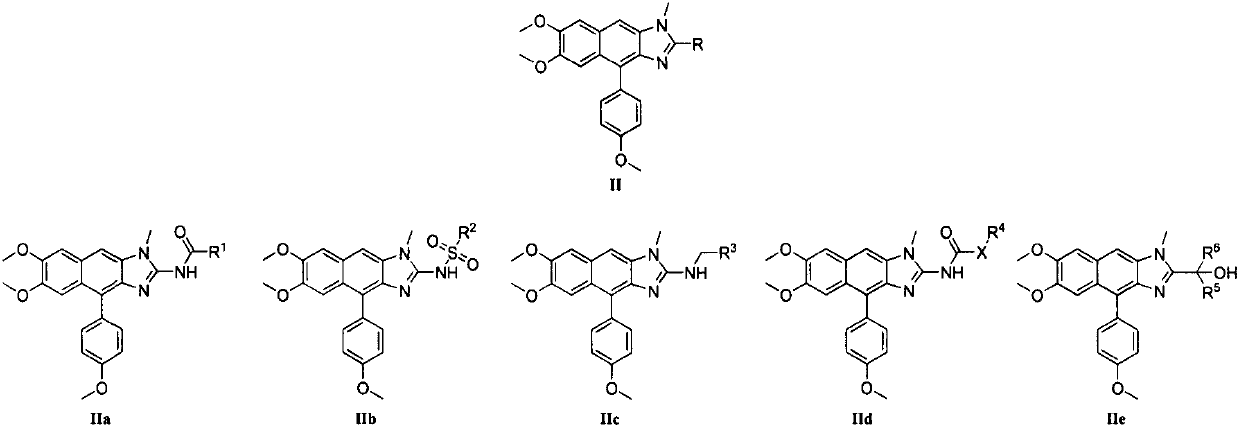
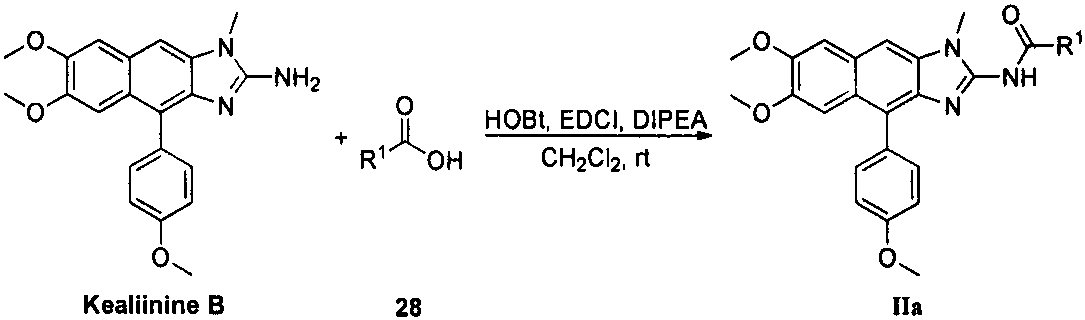
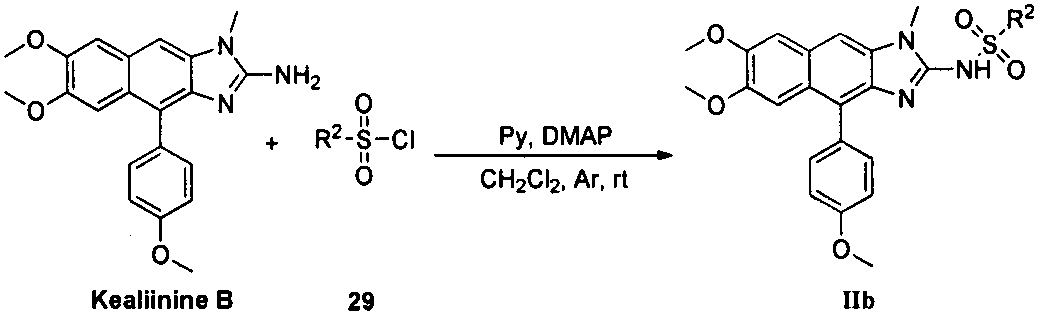
Kealiinine derivative, preparation thereof and application of the derivative in resisting plant viruses and germs
A technology of plant pathogens and derivatives, applied in the direction of chemicals for biological control, botany equipment and methods, applications, etc., can solve the problem that the biological activity of derivatives has not been systematically studied, and the resistance to plant viruses and bacteria has not been systematically studied. Research and reporting issues, to achieve good anti-plant virus and bacteria activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the synthesis of Kealiinine derivative II-1
[0044] Add EDCI (0.19g, 1.00mmol), HOBt (22mg, 0.16mmol), cyclopentyl formic acid (95mg, 0.83mmol) and DIPEA (0.32g, 2.49mmol) in a 100mL round bottom flask, and distill with 30mL The dichloromethane was dissolved, stirred at room temperature for 30 min, added Kealiinine B (0.30 g, 0.83 mmol), protected by argon, stirred at room temperature for 10 h, and monitored by TLC. Add 30mL of water to dilute the reaction solution, separate the layers, extract the aqueous phase with dichloromethane (30mL×3), combine the organic layers, wash with saturated brine, dry over anhydrous sodium sulfate, filter with suction, precipitate under reduced pressure, column chromatography (V (Petroleum ether): V (ethyl acetate) = 6: 1 → V (petroleum ether): V (ethyl acetate) = 4: 1 → V (petroleum ether): V (ethyl acetate) = 3: 1) , light yellow solid, yield 62%, melting point 196-197°C. 1 H NMR (400MHz, CDCl 3 )δ11.71(s, 1H), 7.42(d...
Embodiment 2
[0045] Embodiment 2: the synthesis of Kealiinine class derivative II-2
[0046] In a 100mL round bottom flask, add Kealiinine B (0.20g, 0.55mmol), DMAP (0.013g, 0.11mmol), triethylamine (0.067g, 0.66mmol), dissolve with 20mL of dichloromethane, argon protection, A solution of methanesulfonyl chloride (0.13 g, 1.13 mmol) in dichloromethane (2 mL) was slowly added dropwise thereto. After the addition was complete, the mixture was stirred at room temperature for 1 h and monitored by TLC. Add 20 mL of water to quench the reaction, separate the layers, extract the aqueous phase with dichloromethane (60 mL×3), combine the organic layers, wash with saturated brine, dry over anhydrous sodium sulfate, filter with suction, precipitate under reduced pressure, and perform column chromatography on the crude product (V (dichloromethane): V (methanol) = 50:1), 0.21 g of a light yellow solid was obtained with a yield of 86% and a melting point of 204-205°C. 1 H NMR (400MHz, CDCl 3 )δ9.76(s,...
Embodiment 3
[0047] Embodiment 3: the synthesis of Kealiinine class derivative II-3 and II-4
[0048] II-3: Kealiinine B (0.52g, 1.43mmol), benzaldehyde (0.23g, 2.17mmol), and toluene (30mL) were added to a 100mL round bottom flask, heated to 110°C, and reacted overnight. Precipitate under reduced pressure, remove toluene, add ethanol (30 mL), add sodium borohydride (0.20 g, 5.26 mmol) in batches under stirring at room temperature, react for 3 h, and monitor by TLC. Add a small amount of water to quench the reaction, remove most of the solvent under reduced pressure, add 50 mL of water, extract with dichloromethane (80 mL×3), combine the organic layers, wash with saturated brine, dry over anhydrous sodium sulfate, suction filter, and reduce pressure Precipitation and column chromatography of the crude product (V (dichloromethane): V (methanol) = 50: 1) gave 0.49 g of a yellow solid with a yield of 76% and a melting point of 112-114°C. 1 H NMR (400MHz, CDCl 3 )δ7.57(d, J=8.4Hz, 2H), 7.30-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com