Method for synthesizing roxadustat intermediate
A technology of roxadustat and intermediates, applied in the field of chemical preparation, can solve the problems of high cost, shortage of 5-bromophthalide supply, large pollution, etc., and achieve the effect of low cost and easy control of reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
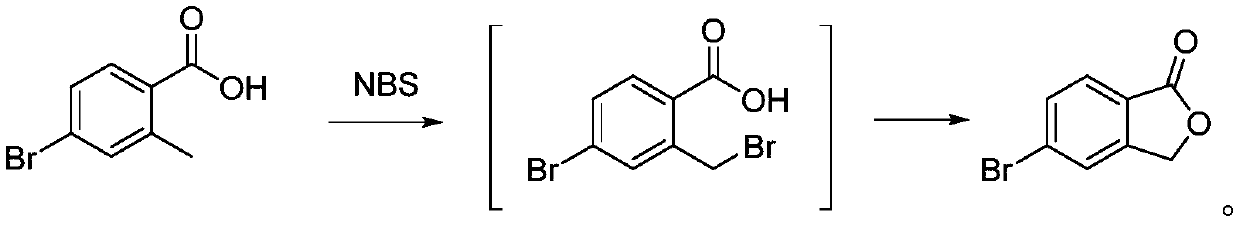
[0024] The purpose of this embodiment is to provide a kind of method of synthesizing roxadustat intermediate, and concrete steps are as follows:
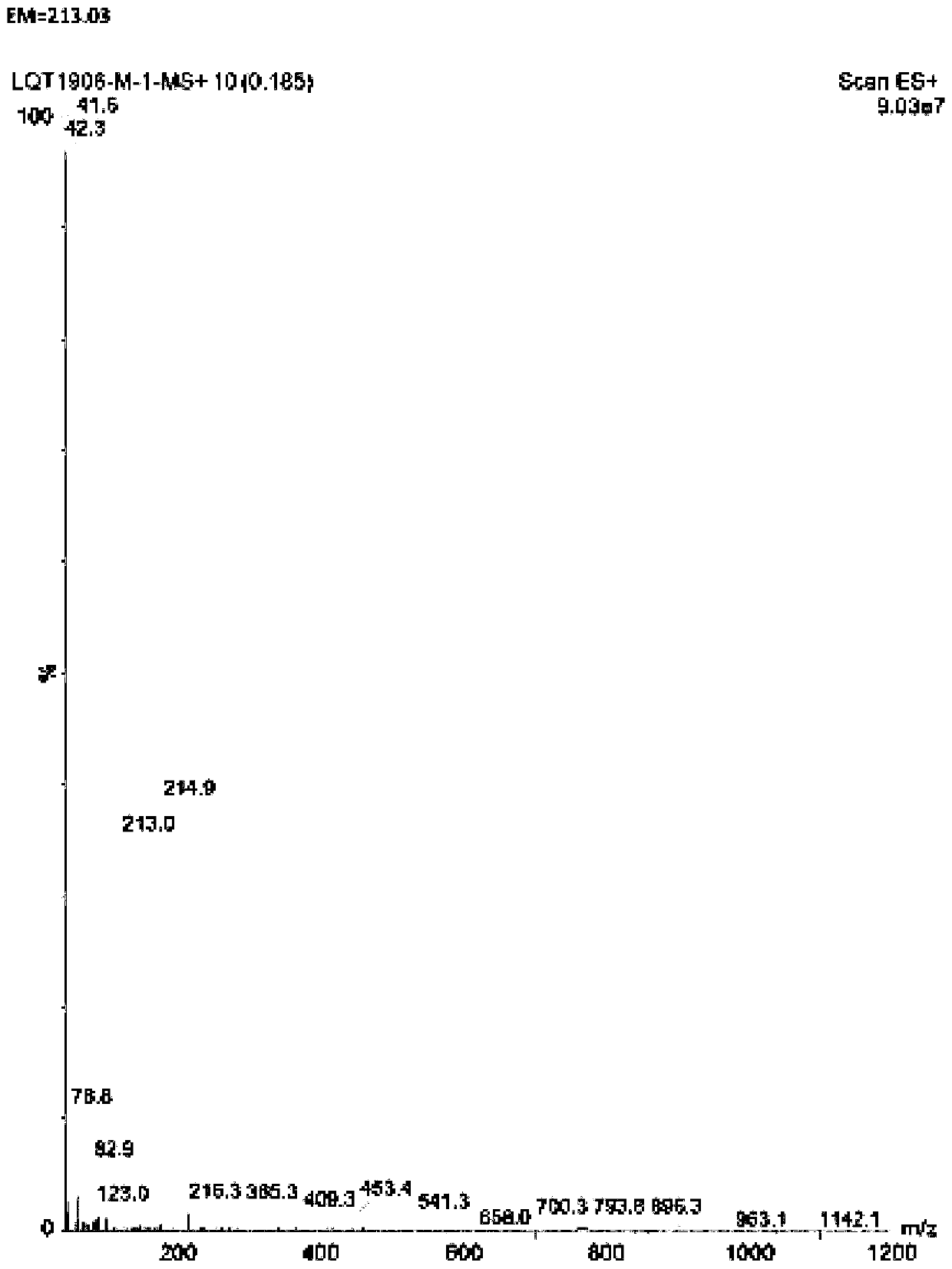
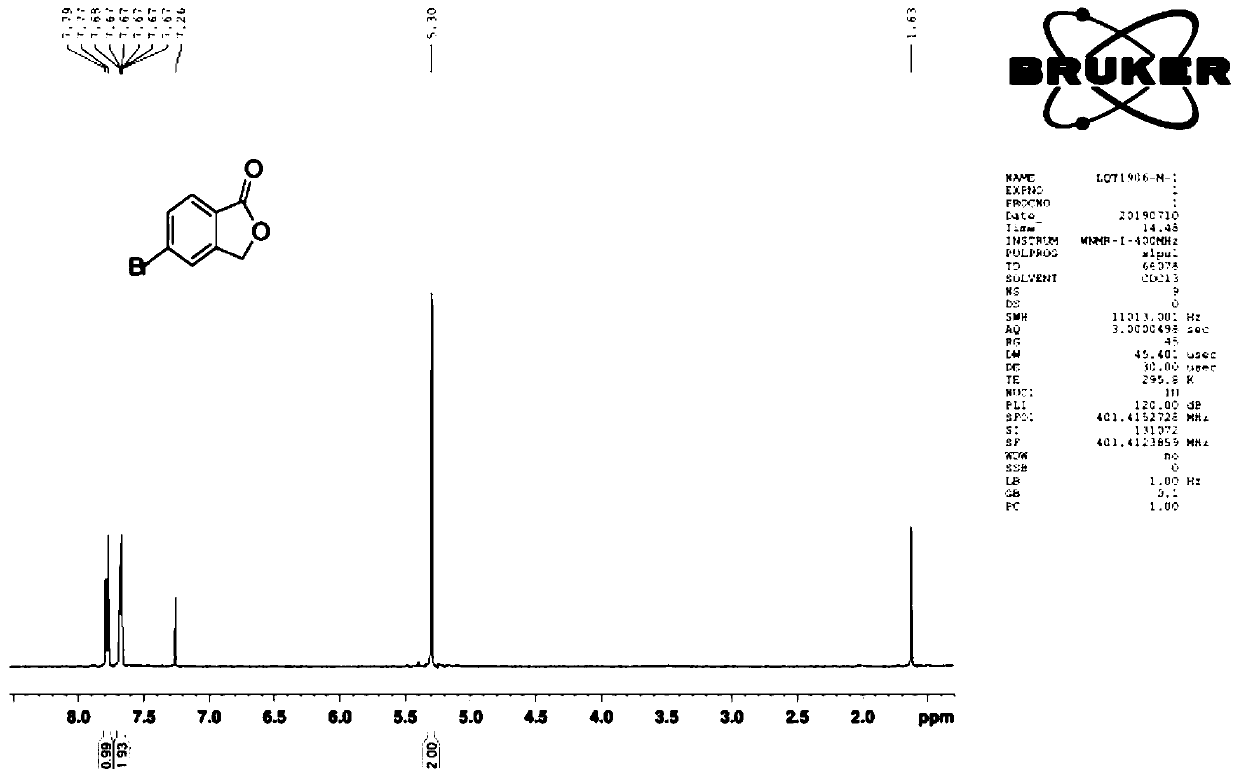
[0025] Add 75.0g (0.35mol) of 4-bromo-2-methylbenzoic acid and 600g of ethylene dichloride to the three-necked reaction flask, mix to obtain mixture one, then add N-bromosuccinimide to mixture one 68.3 g (0.38 mol), 3.3 g (0.02 mol) of azobisisobutyronitrile, mix well to obtain mixture 2, heat mixture 2 to 60°C, and react for 3 h; after the reaction is completed, a reaction solution is obtained, and the reaction solution is used Wash once with 5% aqueous potassium carbonate solution and once with saturated sodium chloride solution, then dry with anhydrous sodium sulfate, and concentrate to dryness to obtain 71.8 g of 5-bromophthalide with a yield of 96.6%. The purity is 98.5 g by liquid chromatography. %.
Embodiment 2
[0027] The purpose of this embodiment is to provide a kind of method of synthesizing roxadustat intermediate, and concrete steps are as follows:
[0028] In the three-necked reaction flask, add 500g (2.3mol) of 4-bromo-2-methylbenzoic acid, 2500g of dichloroethane, and mix to obtain mixture one, and then add 414g of N-bromosuccinimide to mixture one (2.3mol), 19g (0.12mol) of azobisisobutyronitrile, mix well to obtain mixture 2, heat mixture 2 to 60°C for reaction, and react for 5h; Wash once with potassium carbonate aqueous solution and once with saturated sodium chloride solution, then dry with anhydrous sodium sulfate, and concentrate to dryness to obtain 478 g of 5-bromophthalide in a yield of 96.5%. The purity is 98.8% detected by liquid chromatography.
Embodiment 3
[0030] The purpose of this embodiment is to provide a kind of method of synthesizing roxadustat intermediate, and concrete steps are as follows:
[0031] In the three-necked reaction flask, add 400g (1.86mol) of 4-bromo-2-methylbenzoic acid and 4000g of ethylene dichloride, mix to obtain mixture one, then add 398g of N-bromosuccinimide to mixture one (2.24mol), 60g (0.37mol) of azobisisobutyronitrile, mixed evenly to obtain mixture two, the mixture two was heated to 70 ° C for reaction, and the reaction was carried out for 4h; Wash once with potassium carbonate aqueous solution and once with saturated sodium chloride solution, then dry with anhydrous sodium sulfate, and concentrate to dryness to obtain 380 g of 5-bromophthalide in a yield of 95.9%. The purity is 98.3% detected by liquid chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com