Prodrug of mycophenolic acid and production method of prodrug of mycophenolic acid
A liquid preparation and pharmacy technology, applied in the field of medicine, to achieve the effects of good water solubility and good permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
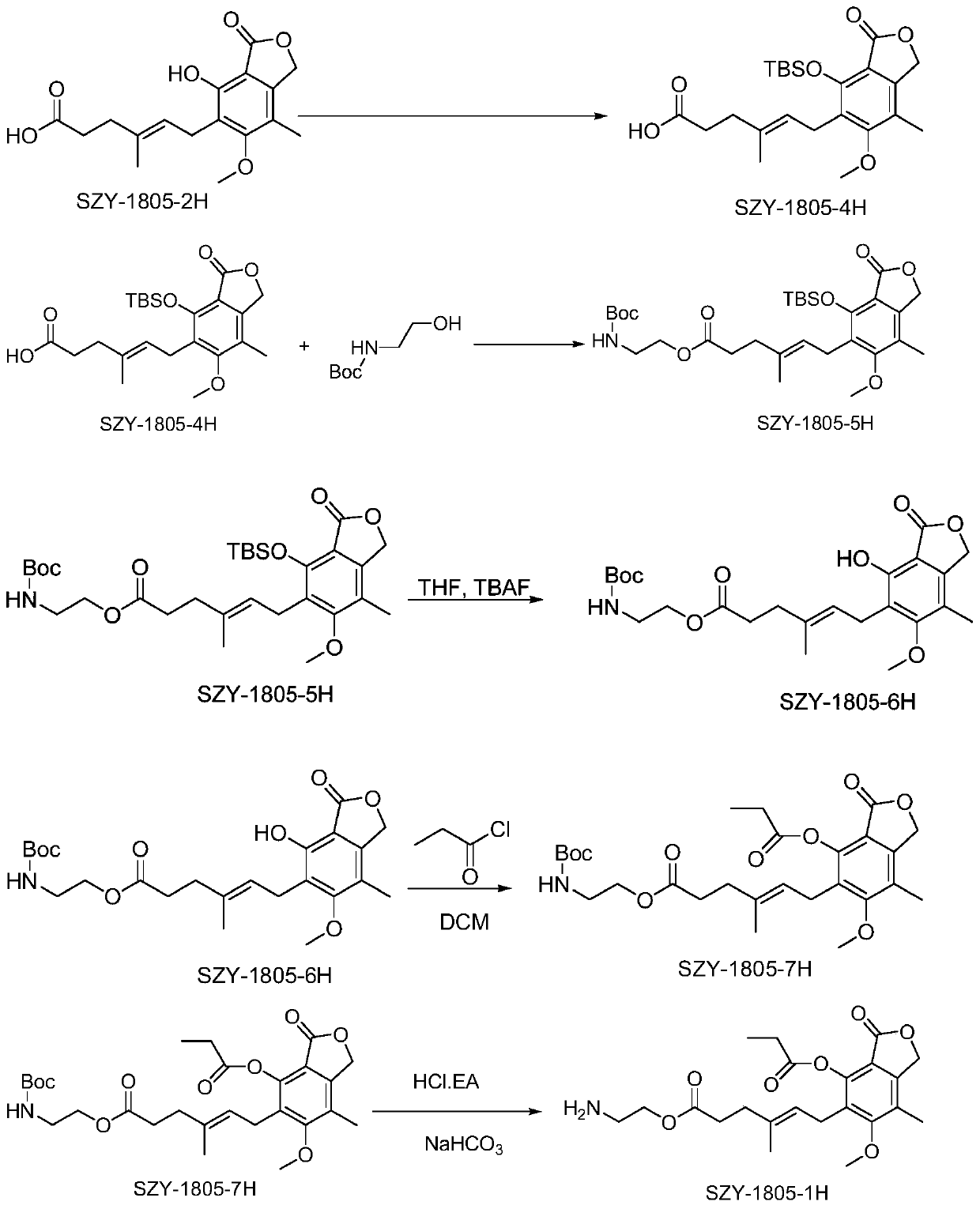
[0059] Embodiment 1, Preparation of (SZY1805-1H) compound
[0060] according to figure 1 The synthetic route diagram shown prepares the above compounds:
[0061] Add SZY-1805-2H (10.0g, 31.22mmol, 1.0eq, MW320.34) in 250ml round bottom flask, add 100mlDMF to dissolve, add imidazole (13.8g, 202.9mmol, 6.5eq, MW68.08) to dissolve, then TBSCl (18.82g, 124.87mmol, 4.0eq, MW150.7) was added in batches, and the reaction was stirred overnight at room temperature. TLC detects that the reaction is complete, and 150ml of EA and 200ml of water are added to the solution to form a layer. The aqueous phase was extracted three times with 50 ml EA, and the organic phases were combined. The organic phase was washed with 1% hydrochloric acid solution and 3-5 times with pure water (DMF was removed). The organic phase was dried with anhydrous sodium sulfate and rotary evaporated to obtain an oily substance. Add 50ml of THF, 50ml of water, and 50ml of acetic acid to the oil, stir at room te...
Embodiment 2
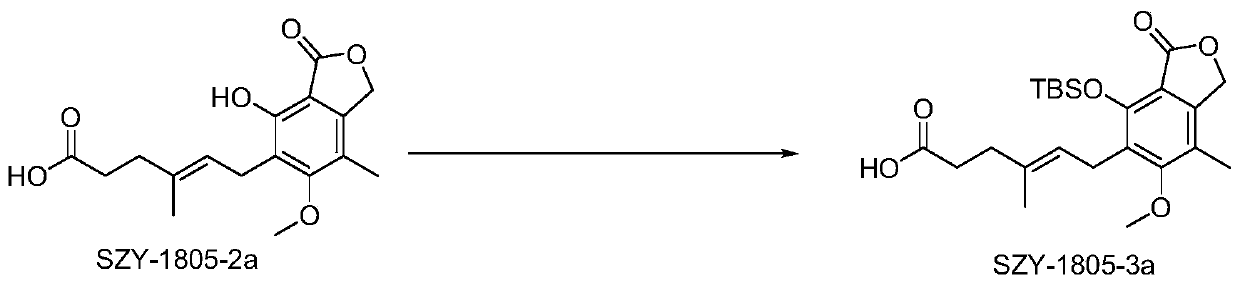
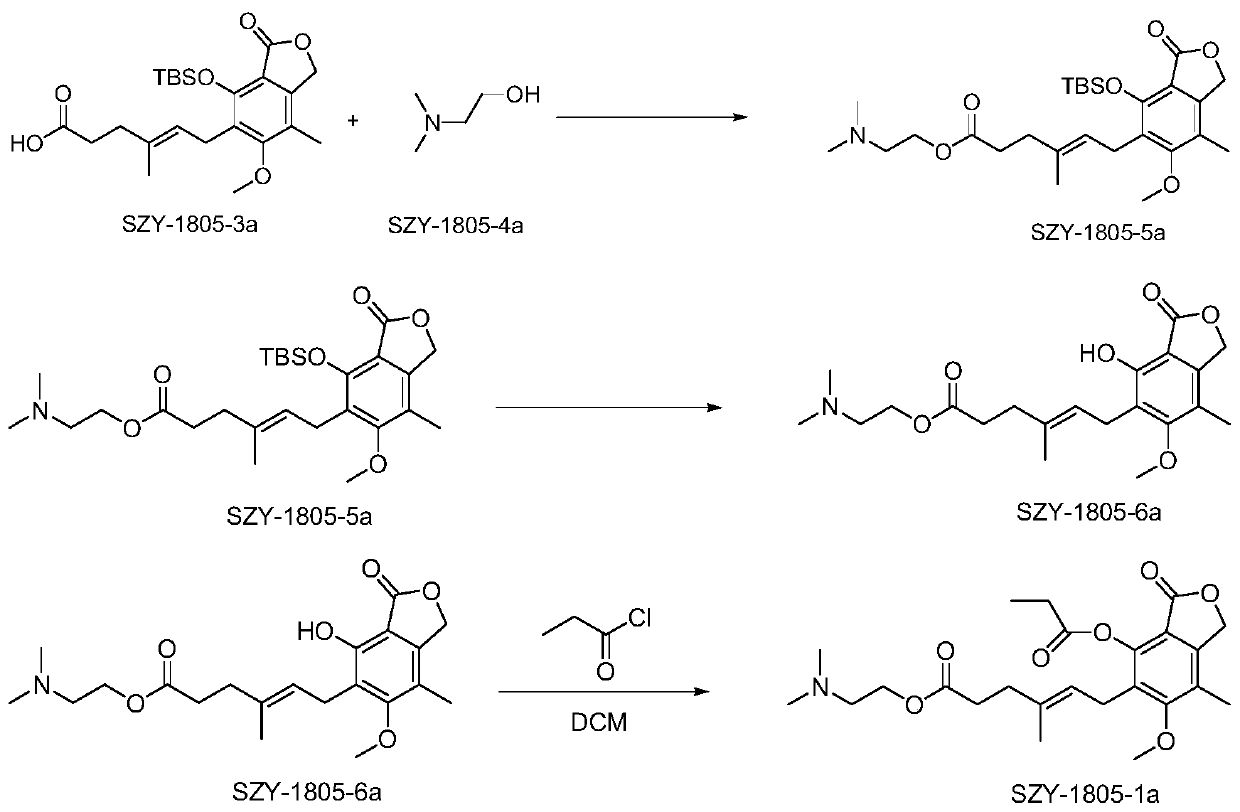
[0069] Embodiment 2, Preparation of (SZY1805-1a) compound
[0070] according to figure 2 The synthetic route diagram shown prepares the above compounds:
[0071] Add SZY-1805-2a (10.0g, 31.22mmol, 1.0eq, MW320.34) in 250ml round bottom flask, add 100mlDMF to dissolve, add imidazole (13.8g, 202.9mmol, 6.5eq, MW68.08) to dissolve, then TBSCl (18.82g, 124.87mmol, 4.0eq, MW150.7) was added in batches, and the reaction was stirred overnight at room temperature. TLC detects that the reaction is complete, and 150ml of EA and 200ml of water are added to the solution to form a layer. The aqueous phase was extracted three times with 50 ml EA, and the organic phases were combined. The organic phase was washed with 1% hydrochloric acid solution and 3-5 times with pure water (DMF was removed). The organic phase was dried with anhydrous sodium sulfate and rotary evaporated to obtain an oily substance. Add 50ml of THF, 50ml of water, and 50ml of acetic acid to the oil, stir at room t...
Embodiment 3
[0078] Embodiment 3, Preparation of (SZY1805-1f) compound
[0079] according to image 3 The synthetic route diagram shown prepares the above compounds:
[0080] Add SZY-1805-2f (10.0g, 31.22mmol, 1.0eq, MW320.34) in 250ml round bottom flask, add 100mlDMF to dissolve, add imidazole (13.8g, 202.9mmol, 6.5eq, MW68.08) to dissolve, then TBSCl (18.82g, 124.87mmol, 4.0eq, MW150.7) was added in batches, and the reaction was stirred overnight at room temperature. TLC detects that the reaction is complete, and 150ml of EA and 200ml of water are added to the solution to form a layer. The aqueous phase was extracted three times with 50 ml EA, and the organic phases were combined. The organic phase was washed with 1% hydrochloric acid solution and 3-5 times with pure water (DMF was removed). The organic phase was dried with anhydrous sodium sulfate and rotary evaporated to obtain an oily substance. Add 50ml of THF, 50ml of water, and 50ml of acetic acid to the oil, stir at room te...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com