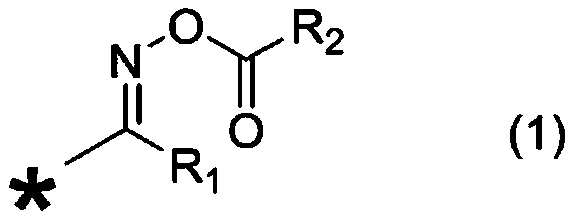
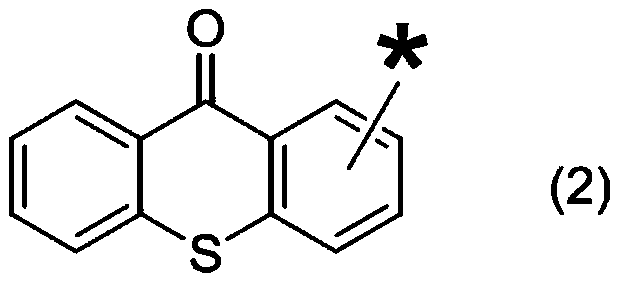
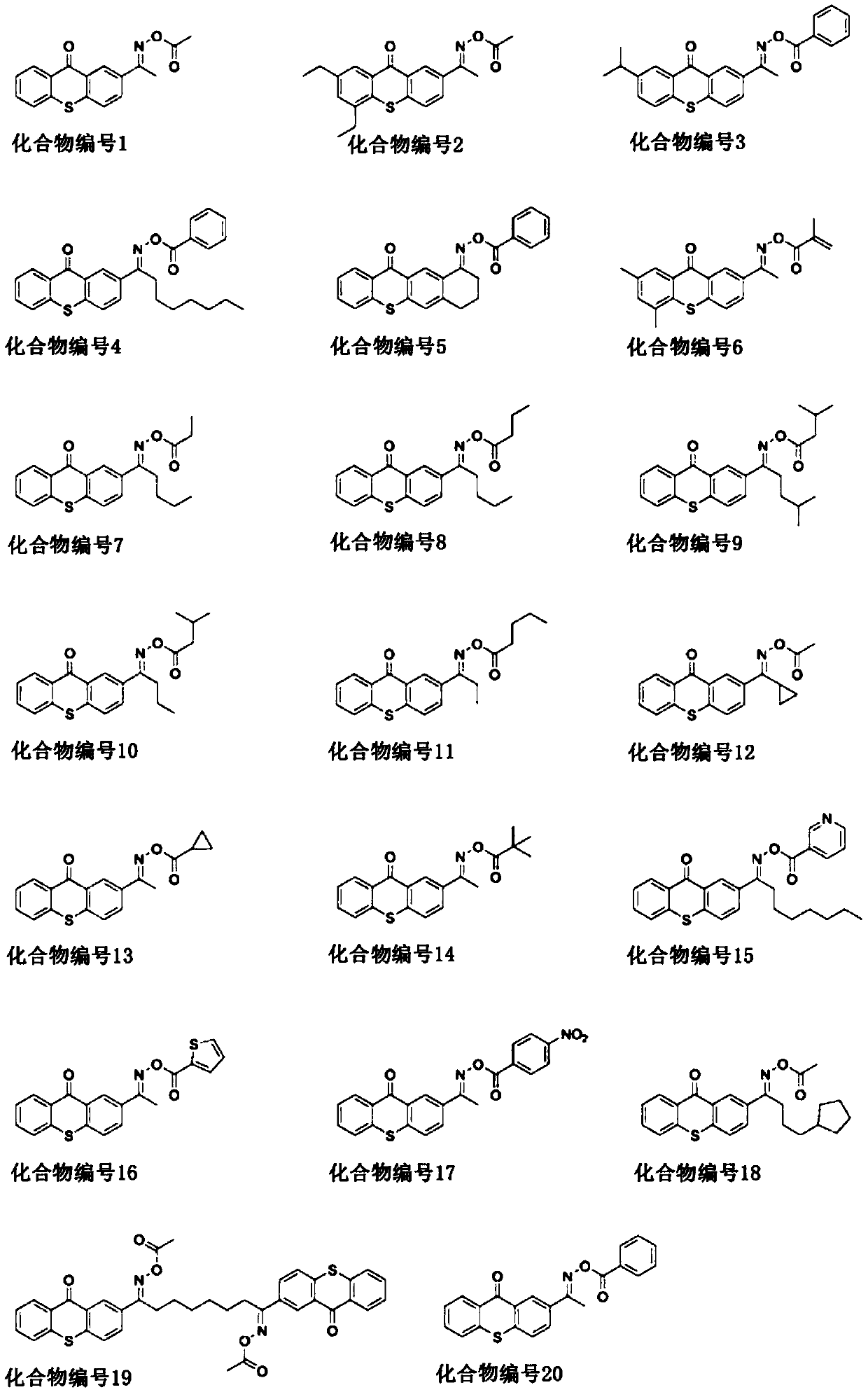
Photocurable composition and electronic component adhesive
A light-curable, electronic component technology, applied in the direction of non-polymer organic compound adhesives, adhesives, adhesive types, etc., can solve the problems of unrealized liquid crystal sealants, low liquid crystal pollution, and damage to moisture resistance reliability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0126] Hereinafter, although an Example demonstrates this invention in detail, this invention is not limited to an Example. In addition, unless otherwise stated, "%" here is a mass basis.
Synthetic example 1
[0128] [Synthesis of 2-acetylthioxanthone]
[0129]
[0130] 2-Acetylthioxanthone was synthesized according to the synthesis sequence described in the existing literature (Material Technology, Vol.27, No.6(2009), pp.242-251).
[0131] [Synthesis of TX-OXE (Compound No. 1)]
[0132]
[0133] A thermometer and a cooling tube were installed in a 200 mL four-neck reaction vessel, and nitrogen gas flow was started at a flow rate of 30 mL / min. 2-Acetylthioxanthone (0.50 g), hydroxylamine hydrochloride (0.20 g), and N,N-dimethylformamide (60 mL) were added to a reaction vessel, and the reaction was carried out at 80° C. for 4 hours. After adding 50 mL of water to stop the reaction, extraction was performed with methyl isobutyl ketone (200 mL), and water washing was performed three times with 50 mL of water. The solvent was removed using an evaporator to obtain an oximate compound of 2-acetylthioxanthone (TX-OX / yellow solid). The yellow solid (crude crystal) obtained here was...
Embodiment 1 and 2
[0142] The curable compounds (B-1, B-2, B-3) shown in the following Table 1 were mixed, heated at 90°C to dissolve the specific compound (A-1) shown in the following Table 1, and cooled until room temperature. Then, after adding and stirring the remaining components in the following Table 1, it was dispersed using a three-roll mill. Then, the photocurable compositions of Examples 1 and 2 were prepared by filtering with a metal sieve (635 mesh). In addition, the numerical value of each component in Table 1 shows a mass part.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap