Preparation method and application of low-molecular-weight heparin-antitumor drug electrostatic complex nano system
An anti-tumor drug and low-molecular-weight heparin technology, which is applied in the field of self-assembled nanosystems of low-molecular-weight heparin-anti-tumor drug electrostatic complexes and its preparation, can solve problems such as bleeding and side effects, and achieve high drug loading and high Effects of safety, good biocompatibility and biosafety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
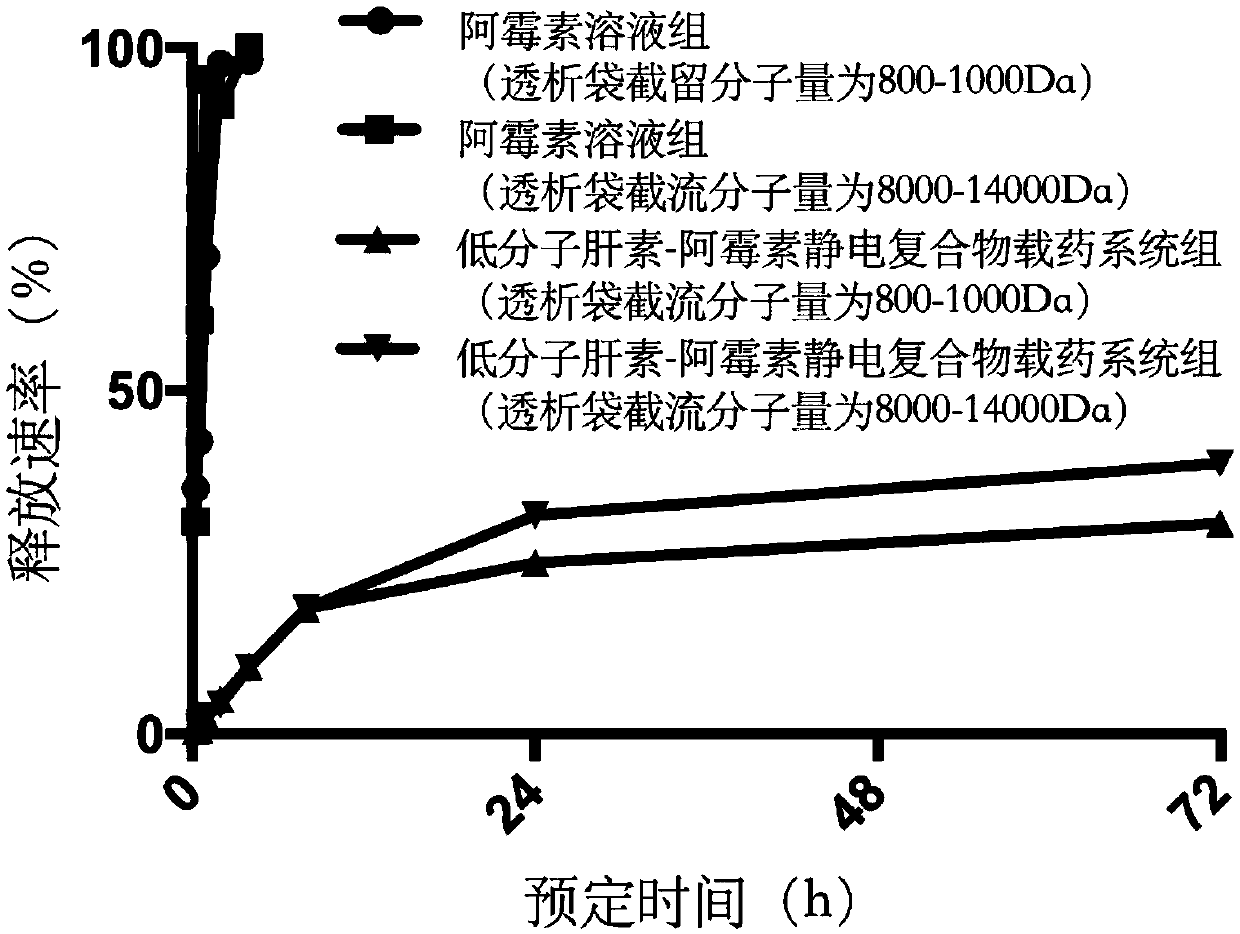
Image
Examples
Embodiment 1
[0064] Example 1: Preparation of Low Molecular Weight Heparin-Adriamycin Electrostatic Complex
[0065] Dissolve low molecular weight heparin (100 mg) in an appropriate amount of deionized water, dissolve adriamycin hydrochloride (10 mg, calculated as adriamycin hydrochloride) in an appropriate amount of deionized water, and dissolve adriamycin hydrochloride The saline solution was quickly added to the low-molecular-weight heparin solution, and stirring was continued at room temperature for 12 hours. The precipitate is separated by centrifugation, and the obtained precipitate is dispersed in dimethyl sulfoxide to obtain a dimethyl sulfoxide solution of the low-molecular-weight heparin-doxorubicin electrostatic complex.
Embodiment 2
[0066] Example 2: Preparation of Low Molecular Weight Heparin-Adriamycin Electrostatic Complex
[0067] Dissolve low molecular weight heparin (100 mg) in an appropriate amount of deionized water, dissolve adriamycin hydrochloride (20 mg, calculated as adriamycin hydrochloride) in an appropriate amount of deionized water, and dissolve adriamycin hydrochloride The saline solution was quickly added to the low-molecular-weight heparin solution, and stirring was continued at room temperature for 12 hours. The precipitate is separated by centrifugation, and the obtained precipitate is dispersed in dimethyl sulfoxide to obtain a dimethyl sulfoxide solution of the low-molecular-weight heparin-doxorubicin electrostatic complex.
Embodiment 3
[0068] Example 3: Preparation of Low Molecular Weight Heparin-Adriamycin Electrostatic Complex
[0069] Low-molecular-weight heparin (100 mg, calculated as doxorubicin hydrochloride) was dissolved in an appropriate amount of deionized water, and adriamycin hydrochloride (50 mg) was dissolved in an appropriate amount of deionized water, and the doxorubicin hydrochloride was dissolved under stirring. The saline solution was quickly added to the low-molecular-weight heparin solution, and stirring was continued at room temperature for 12 hours. The precipitate is separated by centrifugation, and the obtained precipitate is dispersed in dimethyl sulfoxide to obtain a dimethyl sulfoxide solution of the low-molecular-weight heparin-doxorubicin electrostatic complex.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com