Fused imidazo-piperidine JAK inhibitor compound
A technology of compound and pharmaceutical composition, which is applied in the field of preparing the compound and can solve the problem that the disease is not controlled by conventional therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0187] The following synthetic and biological examples are provided to illustrate the invention and are not to be construed as limiting the scope of the invention in any way. In the examples below, the following abbreviations have the following meanings unless otherwise indicated. Abbreviations not defined below have their generally accepted meanings.
[0188] CAN = acetonitrile
[0189] DCC = Dicyclohexylcarbodiimide
[0190] DIPEA=N,N-Diisopropylethylamine
[0191] DMAc = dimethylacetamide
[0192] DMF=N,N-Dimethylformamide
[0193] DMSO = dimethyl sulfoxide
[0194] EtOAc = ethyl acetate
[0195] HATU = N,N,N',N'-tetramethyl-O-(7-azabenzotriazol-1-yl)urea salt of hexafluorophosphate
[0196] LDA = lithium diisopropylamide
[0197] Min=minute
[0198] MTBE = methyl tert-butyl ether
[0199] NBS = N-bromosuccinimide
[0200] NMP = N-methyl-2-pyrrolidone
[0201] RT = room temperature
[0202] THF = Tetrahydrofuran
[0203] Bis(pinacolyl)diboron=4,4,5,5,4',4',5',...
example 1
[0256] Example 1: 1-(2-(6-(2-Ethyl-5-fluoro-4-hydroxyphenyl)-4-fluoro-1H-indazol-3-yl)-1,4,6,7- Tetrahydro-5H-imidazo[4,5-c]pyridin-5-yl)-2-morpholinoethan-1-one (1)
[0257]
[0258] 5-Ethyl-2-fluoro-4-(4-fluoro-3-(4,5,6,7-tetrahydro-1H-imidazo[4,5-c]pyridine-2- Base)-1H-indazol-6-yl)phenol (6) 2HCl (100g, 214mmol), 2,5-dioxopyrrolidinyl-1-2-morpholinoacetic acid ester (7') (67.2g, 278 mmol) and DIPEA (69 g, 534 mmol) in DMF (600 mL) for 12 h and filtered. By reverse phase chromatography (Agela FLEXATM FS-1L instrument; 2kg Agela C18 DAC column; 200g sample is dissolved in DMF (900mL); Flow rate 300mL / min; Solvent A water, solvent B ACN; Gradient (B%, time (mins): 0 / 15, 0-40 / 45, 40 / 50) Purification of the solution afforded the title compound (50.0 g, 44.8% yield) as a light yellow solid. (m / z): [M+H ] + C 27 h 28 f 2 N 6 o 3 Calculated value: 523.22; Experimental value: 523.0. 1 H NMR (400MHz,MeOD)δ7.22(s,1H),6.80-6.96(m,3H),4.68-4.78(m,2H),3.96(s,2H),3.65-3.95(m...
example 2
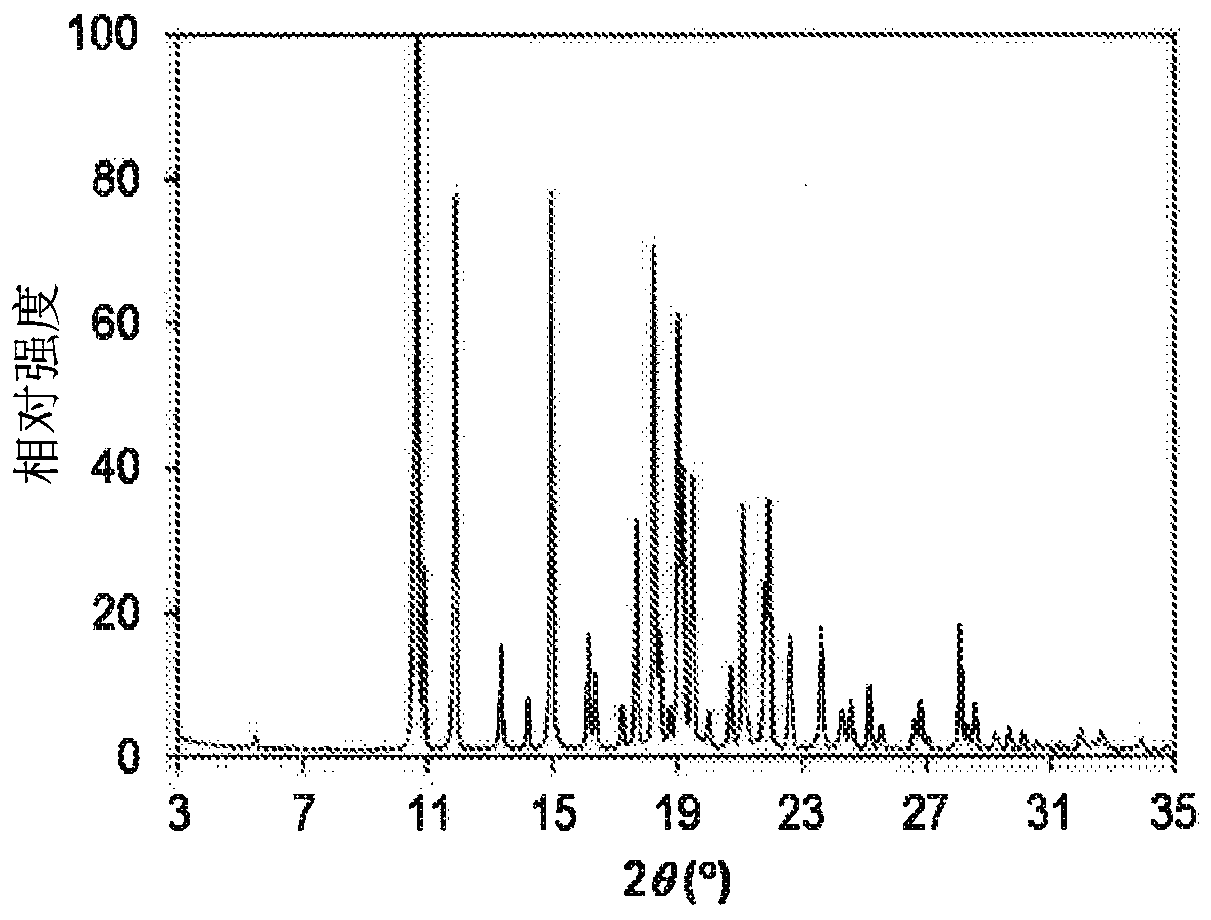
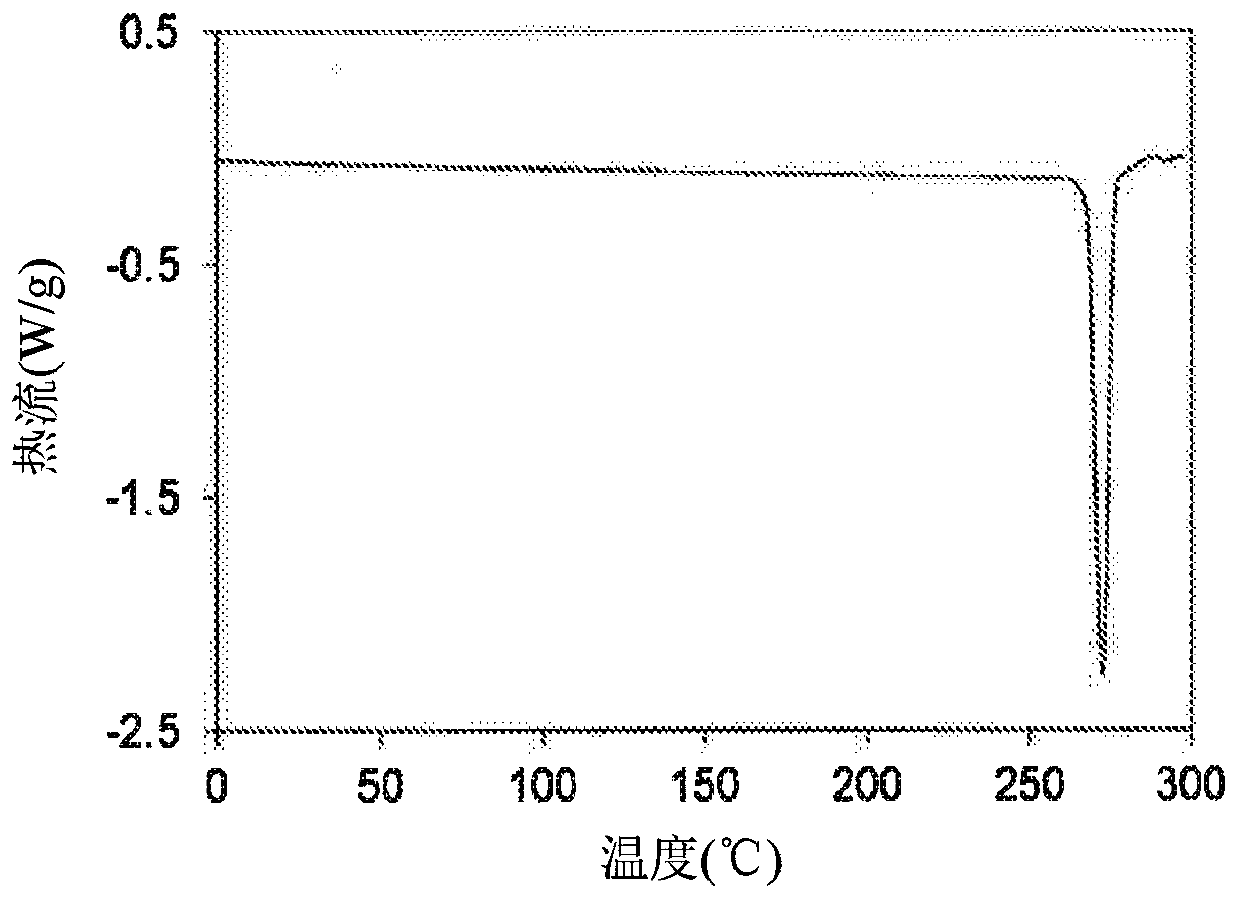
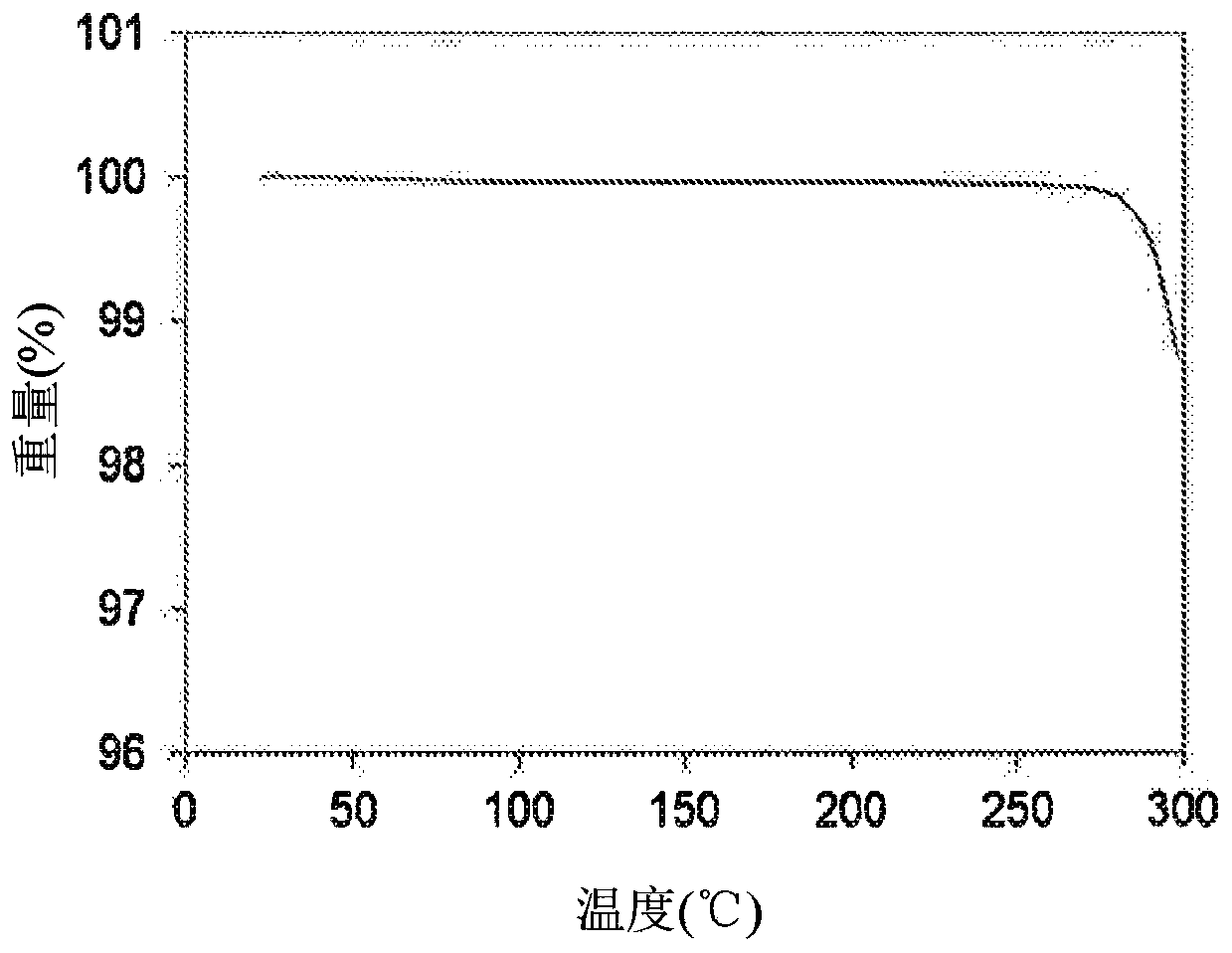
[0259] Example 2: Crystalline 1-(2-(6-(2-ethyl-5-fluoro-4-hydroxyphenyl)-4-fluoro-1H-indazol-3-yl)-1,4,6,7 -Tetrahydro-5H-imidazo[4,5-c]pyridin-5-yl)-2-morpholinoethan-1-one (1) Form 1
[0260] To a 250 mL flask was added 1-(2-(6-(2-ethyl-5-fluoro-4-hydroxyphenyl)-4-fluoro-1H-indazol-3-yl)-1,4,6, 7-tetrahydro-5H-imidazo[4,5-c]pyridin-5-yl)-2-morpholinoethan-1-one (1), the product of Example 1 (5 g) and ethanol (50 mL), And the reaction mixture was stirred at 50-80 °C for 10 minutes, and then ACN (75 mL) was added slowly at 50-80 °C, followed by the seed crystals of Example 3. The reaction mixture was stirred at 20-25°C for 18 hours. The resulting solid was collected by filtration and dried under vacuum at 50 °C for 18 hours to afford the title compound Form 1 (3.6 g, 72% yield)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com