Human epidermal growth factor receptor inhibitor and preparation method and application thereof
A technology of selecting compounds, applied in the field of medicine, can solve the problems of poor metabolic stability and no drugs available
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
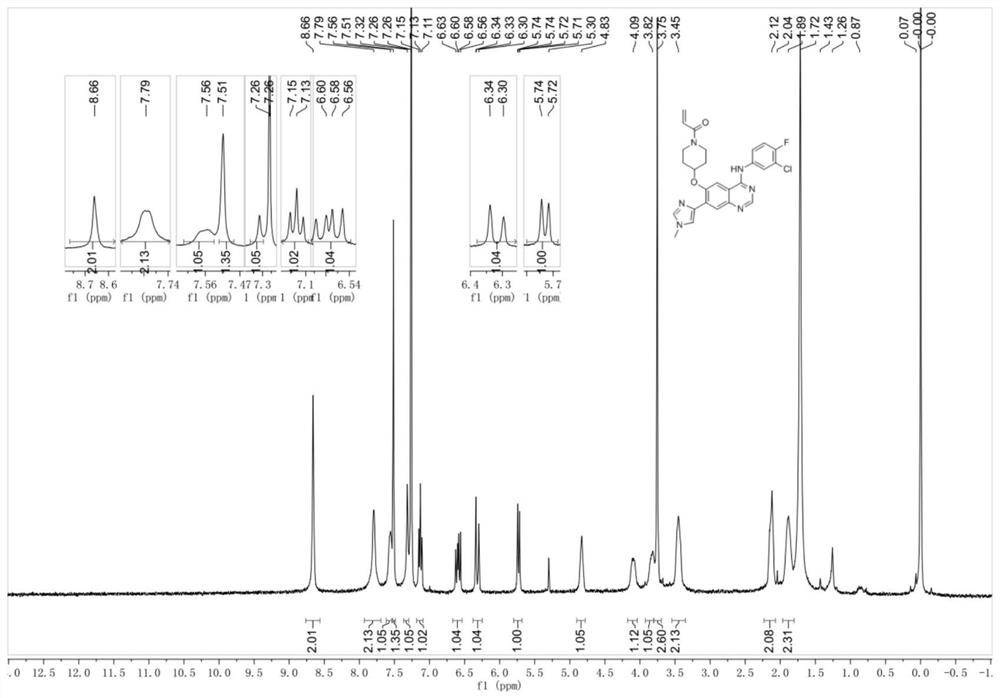
[0132] 1-(4-((4-((3-Chloro-4-fluorophenyl)amine)-7-(1-methyl-1H-imidazol-4-yl)quinazolin-6-yl)oxy) piperidin-1-yl)prop-2-en-1-one
[0133]
[0134] Step 1: Synthesis of 5-((1-((benzyloxy)carbonyl)piperidin-4-yl)oxy)-4-bromo-2-nitrobenzoic acid
[0135]
[0136] Under the ice bath, benzyl 4-hydroxy-1-piperidinecarboxylate (17.80g, 76.0mmol) and DMF (100ml) were added successively to the there-necked flask, and 60% sodium hydride (3.79g, 95.0mmol) was added to the system in batches. ), after reacting at 0 °C for 0.5 h, 4-bromo-5-fluoro-2-nitrobenzoic acid (10.00 g, 37.9 mmol) was added to the above system in batches, and the reaction was complete after 1 h at 0 °C. The pH was adjusted to 3-4 with 2N HCl aqueous solution under ice bath, extracted twice with ethyl acetate, dried over anhydrous sodium sulfate, evaporated to dryness under reduced pressure, and the crude product was directly used in the next step.
[0137] Step 2: Synthesis of (4-(2-bromo-5-(methoxycarbonyl)-...
Embodiment 2
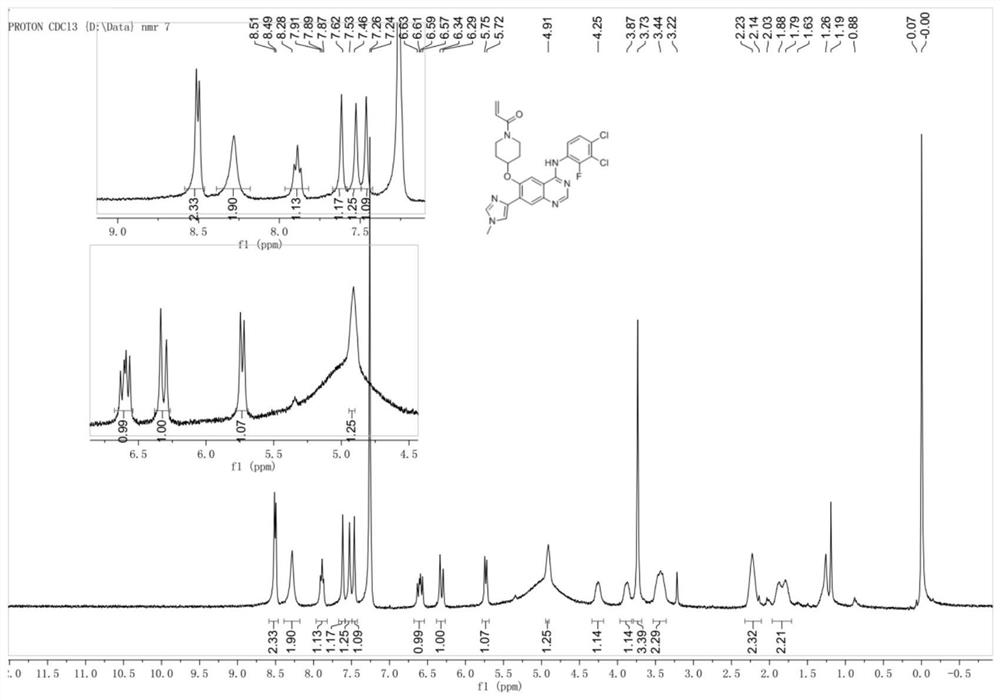
[0165] 1-(4-((4-((3,4-Dichloro-2-fluorophenyl)amine)-7-(1-methyl-1H-imidazol-4-yl)quinazolin-6-yl )oxy)piperidin-1-yl)prop-2-en-1-one
[0166]
[0167] Step 1: Synthesis of 4-((7-bromo-4-((3,4-dichloro-2-fluorophenyl)amine)-quinazolin-6-yl)oxy)piperidine- Benzyl 1-carboxylate.
[0168] Step 2: 4-((4-((3,4-Dichloro-2-fluorophenyl)amine)-7-(1-methyl-1H-imidazol-4-yl)quinazolin-6-yl Synthesis of benzyl )oxy)piperidine-1-carboxylate
[0169]
[0170]At room temperature, add 4-((7-bromo-4-((3,4-dichloro-2-fluorophenyl)amine)-quinazolin-6-yl)oxy)piperidine successively to the single-necked flask - Benzyl 1-carboxylate (0.90 g, 1.45 mmol), 1-methyl-1H-imidazole-4-boronic acid pinacol ester (0.362 g, 1.74 mmol), Pd(dppf)Cl 2 (0.106g, 0.145mmol), 2N K 2 CO 3 (4.0mL), DMF (12mL), heated to 70°C to react overnight, cooled to room temperature after the reaction, 50mL of ethyl acetate was added, washed with aqueous solution (20mL x 2), the aqueous phase was back-extracted once ...
Embodiment 3
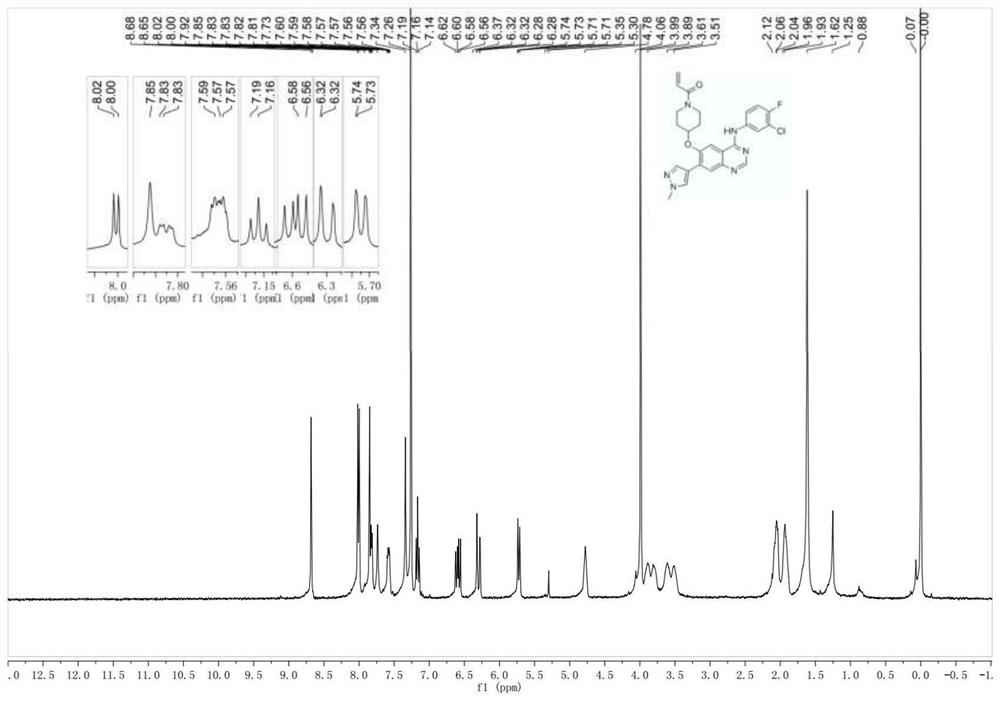
[0178] 1-(4-((4-((3-Chloro-4-fluorophenyl)amine)-7-(1-methyl-1H-pyrazol-4-yl)quinazolin-6-yl)oxy )piperidin-1-yl)prop-2-en-1-one
[0179]
[0180] Step 1: Synthesis of benzyl 4-((7-bromo-4-((3-chloro-4-fluoroaniline)amine)-quinazolin-6-yl)oxy)piperidine-1-carboxylate with reference to Example 1 ester.
[0181] Step 2: 4-((4-((3-Chloro-4-fluoroaniline)amine)-7-(1-methyl-1H-pyrazol-4-yl)quinazolin-6-yl)oxy ) benzyl piperidine-1-carboxylate
[0182]
[0183] At room temperature, add 4-((7-bromo-4-((3-chloro-4-fluoroaniline)amine)-quinazolin-6-yl)oxy)piperidine-1-carboxylic acid successively to the single-necked flask Benzyl ester (0.50 g, 0.85 mmol), 1-methyl-1H-pyrazole-4-boronic acid (0.129 g, 1.03 mmol), Pd(dppf)Cl 2 (0.063g, 0.085mmol), K 2 CO 3 (2N, 3.5mL), DMF (10mL), heated to 70°C to react overnight, cooled to room temperature after the reaction, added 100mL of ethyl acetate, washed with water (50mL x 3), dried over anhydrous sodium sulfate, evaporated to dryn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com