Crystal form of pyridylaminopyrimidine derivative and preparation method thereof
A crystal form and amine-based technology, applied in the field of medicinal chemistry, can solve the problems of different solubility, density, stability and hygroscopicity, which affect the quality stability of raw materials and preparations, clinical efficacy and safety, etc., and achieve simple preparation process , good stability and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1 Preparation of crystal form of compound of formula (I)
[0056] Add formula (I) compound N-{2-{[2-(dimethylamino)ethyl](methyl)amino}-6-(2,2,2-trifluoroethane) to a 250mL three-necked flask Oxy)-5-{[4-(1-methyl-1H-indol-3-yl)pyrimidin-2-yl]amino}pyridin-3-yl}acrylamide 10.0g, add acetone 140mL, magnetic Stir, protect with argon, heat up in an oil bath to 50-56°C to completely dissolve the solid, continue to keep warm and stir for 20 minutes after dissolving, then add 70mL of n-heptane dropwise over 40 minutes, and naturally cool down to 38°C in the oil bath , and crystallized at 38°C for 30 minutes, then naturally cooled to 20°C in an oil bath and continued to stir and crystallize for 1 hour, filtered with suction, rinsed with n-heptane, dried in a vacuum oven at 50°C for 48 hours, and weighed to obtain the formula ( I) Compound crystal form 6.38g, yield 63.8%.
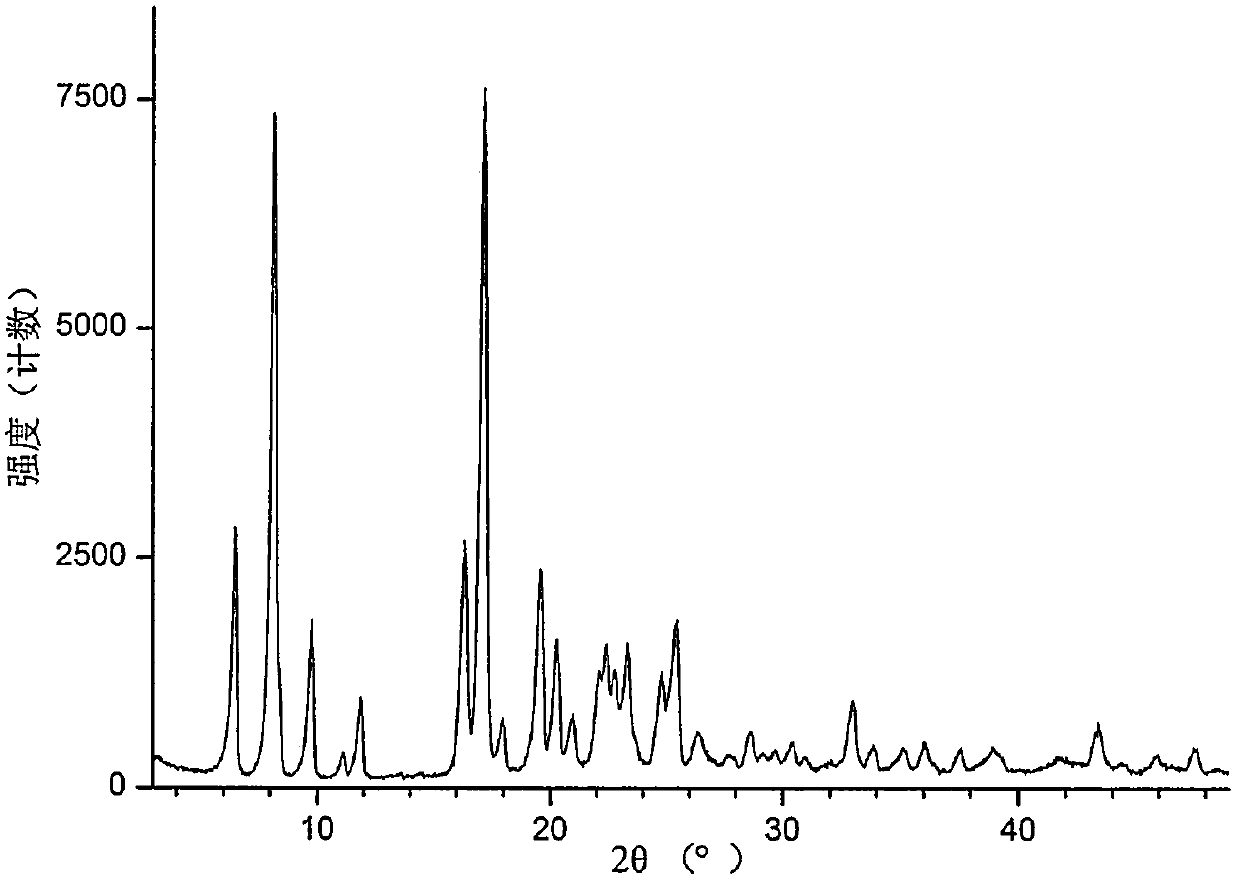
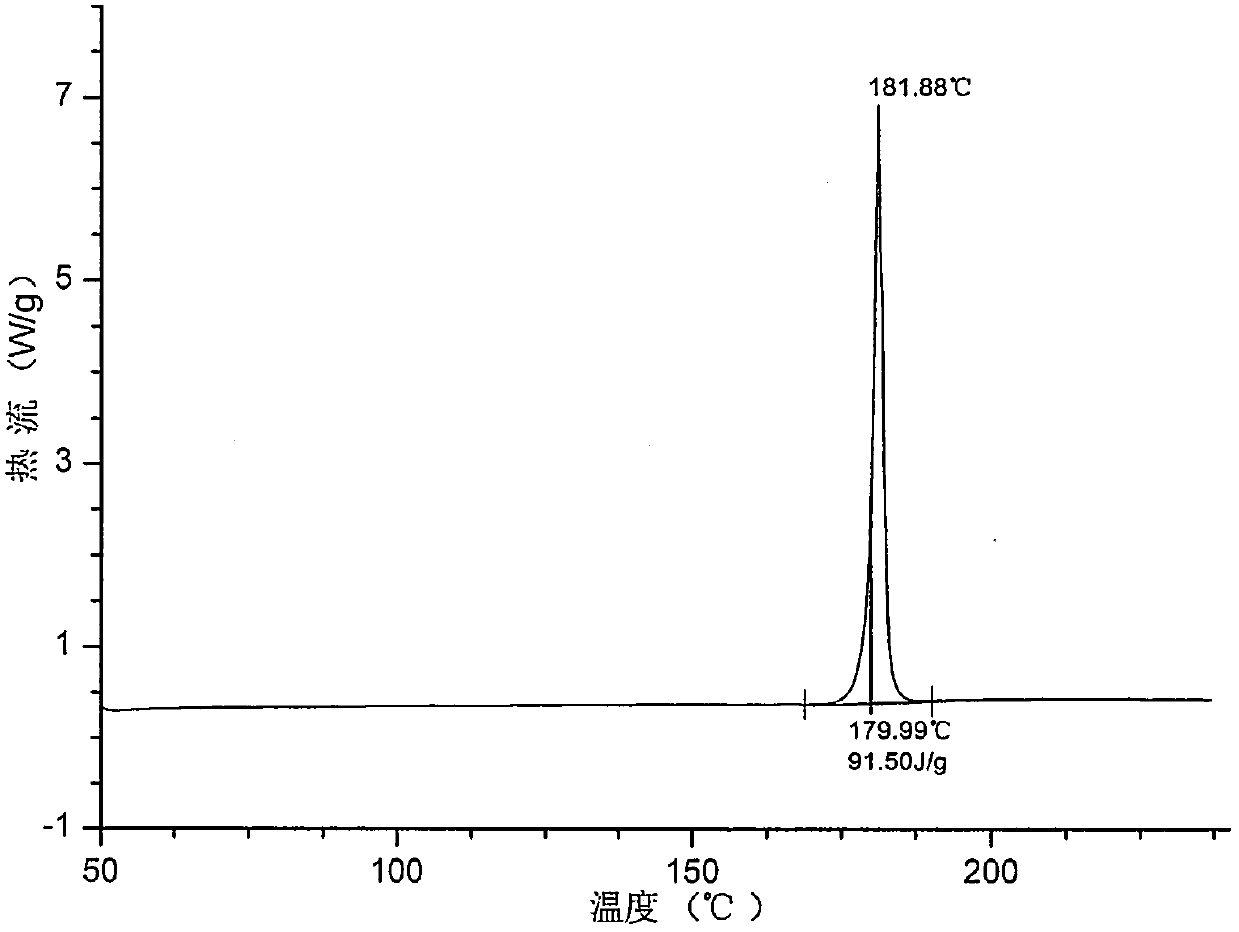
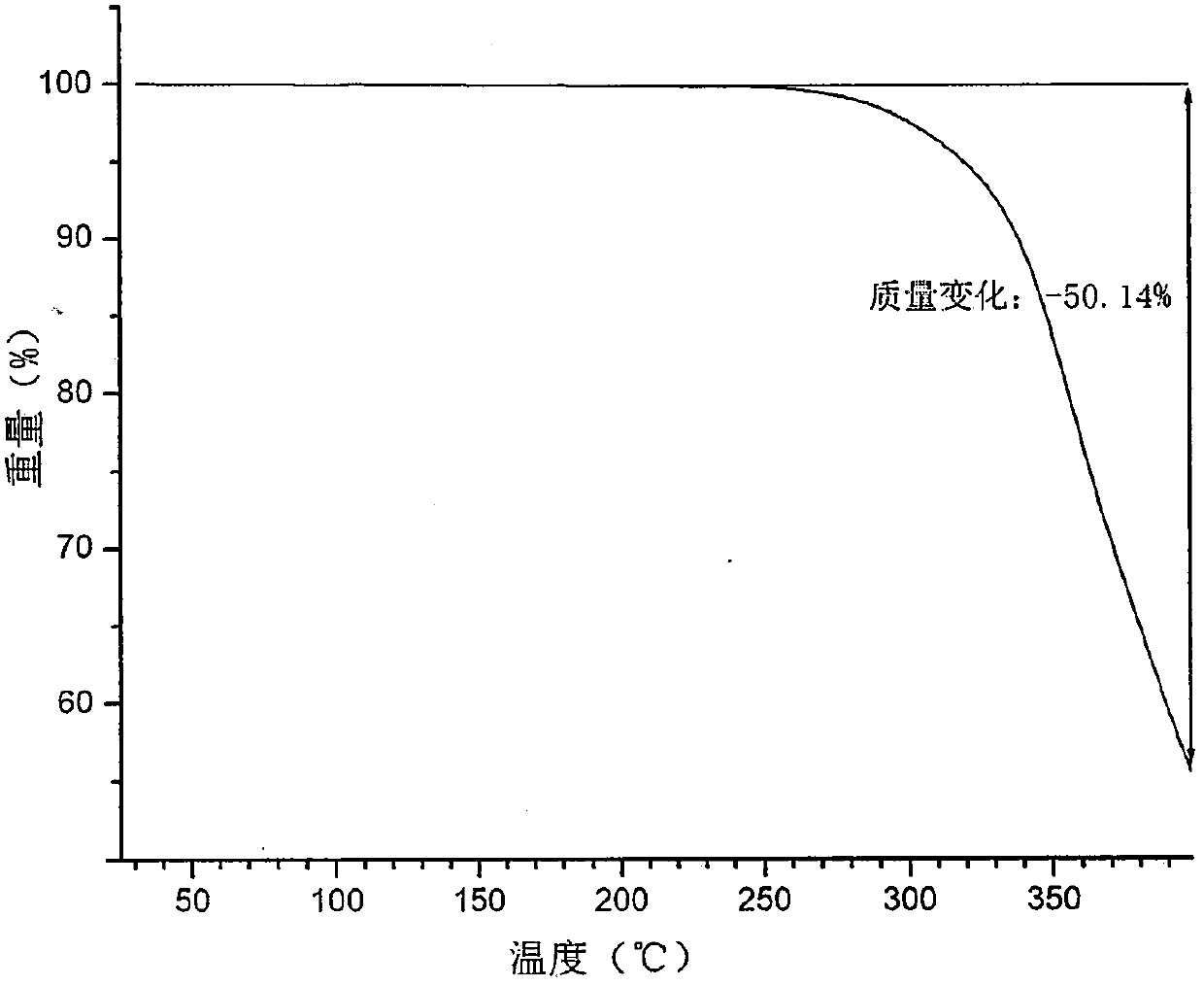
[0057] Its XRPD spectrum, DSC spectrum, TGA spectrum are respectively as follows figure 1 , ...
Embodiment 2
[0060] Embodiment 2 Preparation of crystal form of compound of formula (I)
[0061] Add formula (I) compound N-{2-{[2-(dimethylamino)ethyl](methyl)amino}-6-(2,2,2-trifluoroethane) to a 100mL three-necked flask Oxy)-5-{[4-(1-methyl-1H-indol-3-yl)pyrimidin-2-yl]amino}pyridin-3-yl}acrylamide 3.0g, add tetrahydrofuran 15mL, magnetic Stir, protect with argon, heat up to 40-45°C in an oil bath to completely dissolve the solid, keep stirring for 10 minutes after dissolution, then add 30 mL of n-heptane dropwise over 15 minutes, keep warm and crystallize at 40-45°C After 18 hours, the temperature was naturally lowered to 20°C in an oil bath and continued to stir and crystallize for 2 hours, then suction filtered and rinsed with n-heptane. It was dried in a vacuum oven at 50° C. for 48 hours, weighed 2.67 g, and the yield was 89%. Its XRPD, DSC, and TGA spectra are basically consistent with the results of Example 1.
Embodiment 3
[0062] Embodiment 3 Preparation of crystal form of compound of formula (I)
[0063] Add formula (I) compound N-{2-{[2-(dimethylamino)ethyl](methyl)amino}-6-(2,2,2-trifluoroethane) to a 250mL three-necked flask Oxy)-5-{[4-(1-methyl-1H-indol-3-yl)pyrimidin-2-yl]amino}pyridin-3-yl}acrylamide 10.0g, add tetrahydrofuran 50mL, magnetic Stir, protect with argon, heat up to 40-45°C in an oil bath to completely dissolve the solid, continue to heat and stir for 15 minutes after dissolving, then add 150mL of methyl tert-butyl ether dropwise over 2 hours, at 40-45°C Insulated and crystallized for 18 hours, then naturally cooled to 20°C in an oil bath and continued to stir and crystallize for 2 hours, filtered with suction, and rinsed with methyl tert-butyl ether. Dry in a vacuum oven at 50° C. for 48 hours, weigh 6.3 g, and yield 63%. Its XRPD, DSC, and TGA spectra are basically consistent with the results of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com