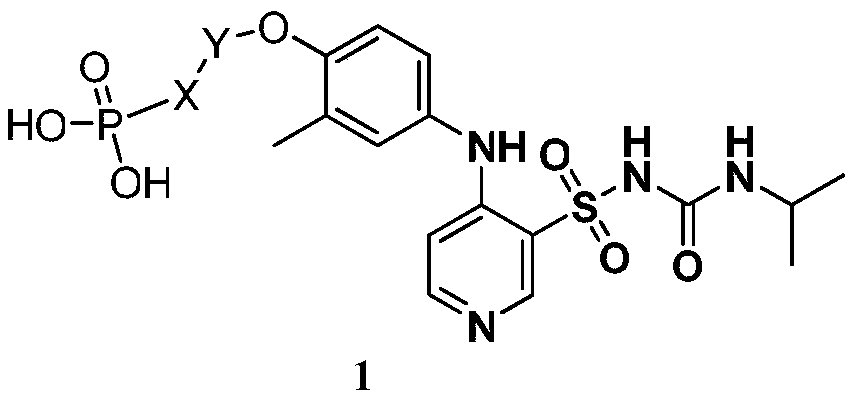
Pyridine sulfonamide phosphate compound as well as preparation method and application thereof
A technology for pyridine sulfonamide phosphate and compounds, which is applied in the field of pyridine sulfonamide phosphate compounds, can solve the problems of not effectively improving water solubility and difficulty in preparation production, and achieve the advantages of convenient preparation of preparations, high stability and high solubility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
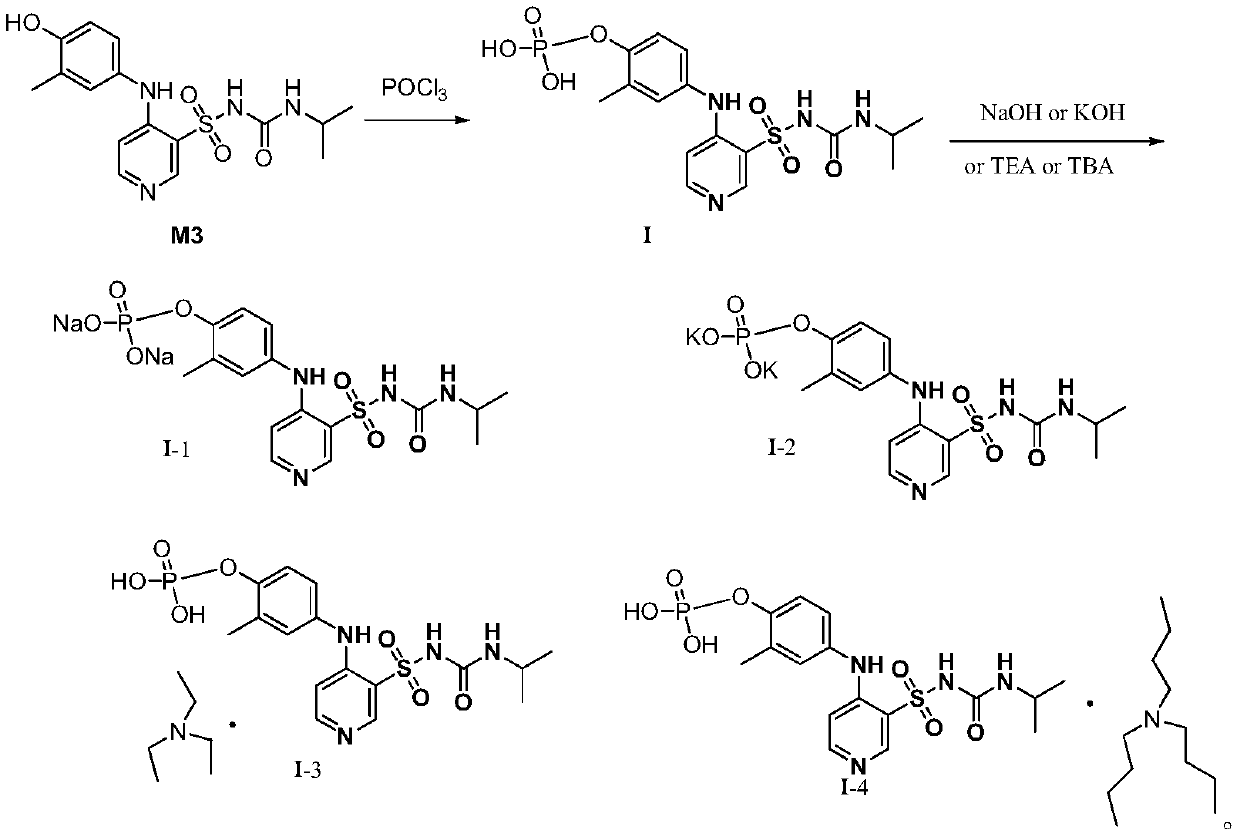
[0027] The preparation of embodiment 1 formula I compound
[0028] Step 1: Preparation of the compound of formula 3
[0029] Add absolute ethanol (300mL) and compound 2 (18.5g, 0.15mol, 1.05eq) into a 500mL reaction flask, stir and heat to about 75°C, add compound 1 (27.5g, 0.143mol, 1.0eq) in batches, add After the completion, keep the temperature and stir the reaction for 1h, and a solid precipitates out. The temperature was lowered to 20-25° C., sodium carbonate (15.9 g, 0.15 mol, 1.5 eq) was added, and paraformaldehyde (15 g, 0.5 mol, 5 eq) was added in batches under stirring. After the addition, the temperature was raised to an internal temperature of 80-85°C for 2 hours, then slowly lowered to an internal temperature of 20-25°C, filtered, and dried to obtain a light yellow filter cake as the compound of formula 3 (36.5g, yield 81%), MS :280[M+1].
[0030] Step 2: Preparation of the compound of formula M3
[0031] Acetone (200mL), compound of formula 3 (35g, 0.11mol, ...
Embodiment 14
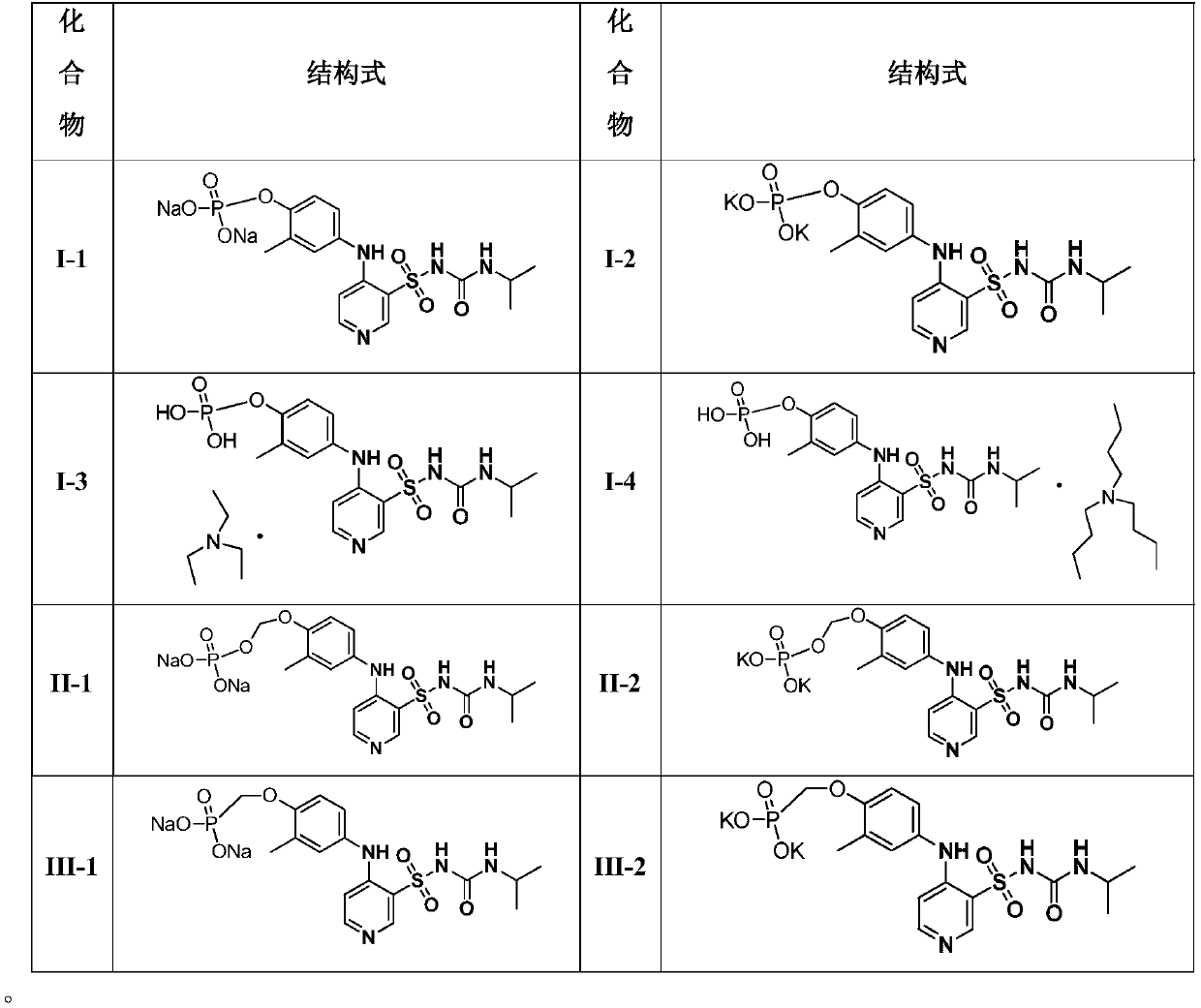
[0083] Embodiment 14 solubility comparison
[0084] Solubility comparison of torasemide, M3, I, I-1, I-2, I-3, I-4, II, II-1, II-2, III, III-1 and III-2 , the result is as follows:
[0085] Table 2. Comparison of water solubility of compounds
[0086]
[0087]
[0088] The results of the solubility experiment show that the solubility of the pyridine sulfonamide phosphate compounds in Examples 1-11 is better than that of torasemide and M3, and has better druggability.
Embodiment 15
[0089] Embodiment 15 diuretic effect comparison
[0090] SD male rats (body weight 180±20g) were randomly divided into 14 groups, 3 rats in each group, and each rat was given 30 mL / kg normal saline by intragastric administration. After intragastric administration of normal saline, except for the blank control group, each group was given a drug (10mg / kg, ig, 1mg / mL, formulation prescription: dissolved in normal saline), and the 4h urination was collected. The results are shown in Table 3:
[0091] Table 3. Comparison of urine output of different compounds
[0092] compound Dosage and method of administration Urine output (mL / kg, 4h) blank control -- 19.2 torsemide 10mg / kg, ig 105.3 M3 10mg / kg, ig 107.2 Compound I 10mg / kg, ig 121.4 Compound I-1 10mg / kg, ig 127.6 Compound I-2 10mg / kg, ig 119.8 Compound I-3 10mg / kg, ig 123.4 Compound I-4 10mg / kg, ig 125.6 Compound II 10mg / kg, ig 119.3 Compound ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com